doi: 10.56294/saludcyt2024.962
REVIEW
Efficacy of wearable cardiac monitoring devices versus traditional methods in detecting atrial fibrillation: a systematic review and meta-analysis
Eficacia de los dispositivos portátiles de monitorización cardíaca frente a los métodos tradicionales en la detección de fibrilación auricular: una revisión sistemática y un metanálisis
Galo Fernando Tulcanaza Ochoa1 ![]() *, Paulina Elizabeth Cisneros Clavijo2
*, Paulina Elizabeth Cisneros Clavijo2 ![]() *, Javier Lizandro Meza Tonato3
*, Javier Lizandro Meza Tonato3 ![]() *, Paola Gissela
Placencia Guartatanga4
*, Paola Gissela
Placencia Guartatanga4 ![]() *, Mónica Paulina Manzano Vela5
*, Mónica Paulina Manzano Vela5 ![]() *, Franklin Isaac Nieto Nuñez6
*, Franklin Isaac Nieto Nuñez6 ![]() *, Adriana Viviana Viñan Andino7
*, Adriana Viviana Viñan Andino7 ![]() *, Néstor Raúl Parrales Ponce8
*, Néstor Raúl Parrales Ponce8 ![]() *
*
1Hospital Misereor de Gualaquiza, Ecuador.
2Pontificia Universidad Católica, Quito, Ecuador.
3Investigador Independiente, Ambato, Ecuador.
4Universidad Católica, Cuenca, Ecuador.
5Universidad de las Fuerzas Armadas, Quito, Ecuador.
6Universidad Regional Autónoma de los Andes, Latacunga, Ecuador.
7Ministerio de Salud Pública, La Gasca, Ecuador.
8Facultad Ciencias de la Salud, Universidad Estatal del Sur de Manabí, Jipijapa, Ecuador.
Cite as: Tulcanaza Ochoa GF, Cisneros Clavijo PE, Meza Tonato JL, Placencia Guartatanga PG, Manzano Vela MP, Nieto Nuñez FI, et al. Efficacy of wearable cardiac monitoring devices versus traditional methods in detecting atrial fibrillation: a systematic review and meta-analysis. Salud, Ciencia y Tecnología. 2024; 4:.962. https://doi.org/10.56294/saludcyt2024.962
Submitted: 18-01-2024 Revised: 18-05-2024 Accepted: 10-09-2024 Published: 11-09-2024
Editor: Dr.
William Castillo-González ![]()
Corresponding author: Galo Fernando Tulcanaza Ochoa *
ABSTRACT
Introduction: atrial fibrillation (AF) is a prevalent arrhythmia with significant health and economic impacts. Traditional detection methods like 12-lead ECGs and Holter monitors are effective but limited by cost and patient compliance. Wearable devices, such as smartwatches and patches, offer a promising alternative for real-time AF detection.
Objective: this systematic review aims to evaluate the efficacy of wearable cardiac monitoring devices compared to traditional methods in detecting AF.
Method: a comprehensive literature search identified studies comparing wearable devices to traditional methods for AF detection. Data on sensitivity, specificity, and usability were extracted and analyzed. A pooled analysis using both fixed-effect and random-effects models assessed overall sensitivity and specificity.
Results: this systematic review analyzed data from 15 studies comparing wearable devices to traditional methods for AF detection. Wearable devices, including smartwatches, patch-type ECGs, and PPG-based technologies, showed high sensitivity and specificity, with the fixed-effect model estimating overall sensitivity at 91,59 % and specificity at 92,13 %. The random-effects model provided slightly higher sensitivity (94,03 %) and specificity (95,96 %). Smartwatches like the Apple Watch with KardiaBand demonstrated up to 97,5 % sensitivity, comparable to insertable cardiac monitors (ICMs). Patch-type ECGs, such as MobiCARE-MC100 and Zio XT, matched Holter monitors in accuracy, with extended monitoring enhancing AF detection. PPG-based technologies, exemplified by the WATCH AF trial, showed 93,7 % sensitivity and 98,2 % specificity. Despite high accuracy, significant heterogeneity among studies highlighted the need for standardized protocols.
Conclusion: wearable devices show high sensitivity and specificity for AF detection, comparable to traditional methods. However, substantial heterogeneity indicates the need for standardized protocols and further research to optimize these technologies for clinical use.
Keywords: Atrial Fibrillation; Electrocardiography; Wearable Electronic Devices; Diagnostic Techniques; Cardiovascular.
RESUMEN
Introducción: la fibrilación auricular (FA) es una arritmia prevalente con significativos impactos en la salud y la economía. Los métodos tradicionales de detección, como los ECG de 12 derivaciones y los monitores Holter, son efectivos pero limitados por su costo y la adherencia del paciente. Los dispositivos portátiles, como relojes inteligentes y parches, ofrecen una alternativa prometedora para la detección en tiempo real de la FA.
Objetivo: esta revisión sistemática tiene como objetivo evaluar la eficacia de los dispositivos portátiles de monitorización cardíaca en comparación con los métodos tradicionales para la detección de FA.
Método: se realizó una búsqueda exhaustiva de la literatura para identificar estudios que compararan dispositivos portátiles con métodos tradicionales para la detección de FA. Se extrajeron y analizaron datos sobre sensibilidad, especificidad y usabilidad. Un análisis conjunto utilizando modelos de efectos fijos y aleatorios evaluó la sensibilidad y especificidad globales.
Resultados: esta revisión sistemática analizó datos de 15 estudios que comparaban dispositivos portátiles con métodos tradicionales para la detección de FA. Los dispositivos portátiles, incluidos relojes inteligentes, ECG tipo parche y tecnologías basadas en PPG, mostraron alta sensibilidad y especificidad, con el modelo de efectos fijos estimando una sensibilidad global del 91,59 % y una especificidad del 92,13 %. El modelo de efectos aleatorios proporcionó una sensibilidad ligeramente mayor (94,03 %) y especificidad (95,96 %). Relojes inteligentes como el Apple Watch con KardiaBand demostraron hasta un 97,5 % de sensibilidad, comparable a los monitores cardíacos insertables (ICM). Los ECG tipo parche, como MobiCARE-MC100 y Zio XT, igualaron la precisión de los monitores Holter, con una monitorización extendida que mejora la detección de FA. Las tecnologías basadas en PPG, ejemplificadas por el ensayo WATCH AF, mostraron una sensibilidad del 93,7 % y una especificidad del 98,2 %. A pesar de la alta precisión, la heterogeneidad significativa entre los estudios destacó la necesidad de protocolos estandarizados.
Conclusión: los dispositivos portátiles muestran alta sensibilidad y especificidad para la detección de FA, comparables a los métodos tradicionales. Sin embargo, la heterogeneidad sustancial indica la necesidad de protocolos estandarizados y más investigación para optimizar estas tecnologías para uso clínico.
Palabras clave: Fibrilación Auricular; Electrocardiografía; Dispositivos Electrónicos Portátiles; Técnicas de Diagnóstico Cardiovascular.
INTRODUCCIÓN
In clinical practice, atrial
fibrillation (AF) is the arrhythmia that is identified most often. In the
United States, the projected number of people affected with AF is 2,3 million,
and as the population ages, that number is predicted to rise to 5,6 million by
2050.(1) Heart failure, stroke, and thromboembolic events are among
the well-known effects of AF. The substantial influence on morbidity,
mortality, and healthcare expenditures may be attributed to these outcomes of
disease development.(1) As a result, AF is not only a grave clinical
issue but also a financial and public health burden.
Although tiredness, dyspnea, palpitations, and chest discomfort are the usual
symptoms of AF, 10–40 % of cases are thought to be asymptomatic.(2)
When subclinical or undetected AF first appears after an acute stroke, it has
significant consequences and is associated with the same risks as symptomatic
AF. It is unclear how arrhythmia and stroke are related, however the Framingham
Study has shown that contemporaneous presentation of stroke and recently
diagnosed AF raises the possibility that cardiac emboli are a significant cause
of stroke.(3) Prophylactic interventions are crucial for preventing
strokes, as shown by the temporal link between AF and stroke. Early preventive
actions may be taken to enhance health outcomes when clinical and subclinical
AF are detected.
The gold standard for diagnosing atrial fibrillation (AF) is interpretation of
a 12-lead electrocardiogram (ECG) by a qualified cardiologist or heart rhythm
expert.(4) According to the American Heart Association/American
Stroke Association’s 2014 recommendations, patients who exhibit an irregular
pulse should first have a 12-lead ECG before being screened for AF with pulse
evaluations performed during routine clinical visits.(5) The
guidelines emphasize the benefits of active screening for individuals over 65,
but they don’t include advice for how often to do it. Comparably, the US
Preventive Services Task Force (USPSTF) released a statement stating that there
is not enough data at this time to assess the value of using an ECG to screen
for AF.(6) The issue is that there are just too many unknowns for
all patients, particularly those who are not high-risk, to benefit from
frequent ECG testing. According to recent studies, two major factors that
contribute to these screening issues are the low prevalence and high prices.(7)
One issue is that since AF is erratic and intermittent, ambulatory ECG
monitoring, which lasts anywhere from 12 hours to 14 days, is only tangentially
indicative of a patient’s experience.(8)
More comfort, convenience, and engagement are possible when it comes to real-time cardiac rhythm monitoring thanks to recent developments in wearable electronics and mobile health technologies. Smartwatches and other wearable technology have a lot of promise for diagnosing cardiac arrhythmias. In recent years, smartwatches have become more and more popular, particularly as a health tool for heart rhythm detection. With only one finger, patients using smartwatches may self-diagnose their cardiac rhythm in about 30 seconds.(9) Early detection of AF reduces the burden on the health care system and guarantees that treatment begins early to avoid further episodes that might negatively affect quality of life. mHealth has provided a way to detect AF outside of the conventional cardiac monitoring system. Electrocardiographic (ECG) or photoplethysmography (PPG) data processing is now used in mHealth technology to identify atrial fibrillation (AF).(10) Although ECG is still the most reliable method for detecting AF, these new technologies are interesting because they can handle near-continuous pulse data passively.(10) PPG and related technologies provide a low-cost, non-invasive way to continuously monitor the heart cycle. Some of the problems with traditional screening techniques for AF detection may be resolved if wearable technology continues to improve in accuracy.
Prior systematic evaluations provide insightful but constrained information about how well wearable technology detects atrial fibrillation (AF). Belani et al. assessed the sensitivity (96,83 %) and varied specificity (Apple: 99,61 %, Samsung: 81,13 %, KardiaBand: 97,98 %) of wrist-worn wearables (Apple Watch, Samsung, and KardiaBand) in detecting AF.(11) But this evaluation did not contrast these wearables with conventional techniques. In their 2020 study, Nazarian et al. examined the diagnostic performance of smartwatches in identifying several cardiac arrhythmias, with results showing 100 %, 95 %, and 97 % overall sensitivity, specificity, and accuracy, respectively.(9) This evaluation just looks at smartwatches; it doesn’t compare them to other conventional monitoring techniques. In light of these drawbacks and the paucity of available research, this systematic review attempts to provide current data about the superiority of a wider variety of wearable cardiac monitoring devices over more conventional techniques for the detection of atrial fibrillation.
METHOD
Study Design
This systematic review and meta-analysis evaluated the efficacy of wearable cardiac monitoring devices compared to traditional methods in detecting atrial fibrillation (AF). The review included studies that assessed the performance of wearable devices against established traditional diagnostic methods.
Sample Selection
The search strategy aimed to identify relevant studies comparing wearable cardiac monitoring devices with traditional methods for AF detection. Searches were conducted in databases including PubMed, Embase, and Cochrane Library using a combination of keywords and MeSH terms. The search terms included “wearable cardiac monitoring, traditional devices, “atrial fibrillation,” “smartwatch,” “ECG,” “Holter monitor,” and “PPG.”
Inclusion Criteria
· Studies comparing wearable cardiac monitoring devices (e.g., smartwatches, wristbands, adhesive ECG patches, patch-type ECG monitors, PPG-based mHealth devices, mobile telemetry, textile Holters) with traditional AF detection methods (e.g., 12-lead ECGs, Holter monitors, implantable cardiac monitors).
· Research articles published in English.
· Studies involving human participants.
· Studies published from January 2019 to July 2024.
· Peer-reviewed journals.
· Studies reporting on sensitivity and specificity for AF detection.
Exclusion Criteria
· Review articles, editorials, conference abstracts, and non-peer-reviewed publications.
· Studies not directly comparing wearable devices with traditional methods
· Studies focusing solely on wearable device validation without comparison to traditional methods.
· Studies with insufficient methodological detail or data on AF detection performance.
Study Method
An initial search was conducted using electronic databases. Abstracts and titles were screened for relevance, and full texts of potentially eligible studies were reviewed in detail. Inclusion and exclusion criteria were applied to select studies for final analysis.
Sample Size
The review included a total of 17 studies meeting the inclusion criteria. Data extraction was performed from these selected studies for analysis.
Quality Assessment
The quality of the included studies was assessed using appropriate tool of Newcastle-Ottawa Scale for observational studies. Quality assessment was conducted independently by two reviewers, with disagreements resolved through discussion or consultation with a third reviewer.
Data Extraction
Data were extracted using a standardized form, capturing key information such as study characteristics (author, year, population), types of wearable and traditional devices, duration of monitoring, sensitivity, specificity, and results.
Data Analysis
Meta-analysis was performed to synthesize quantitative data from the included studies. Sensitivity and specificity estimates were calculated for wearable devices and traditional methods. Statistical heterogeneity among studies was assessed using the I² statistic, with values above 50 % indicating significant heterogeneity. Random-effect models were used to account for variability between studies. Sensitivity analyses and subgroup analyses were conducted to explore sources of heterogeneity and evaluate the robustness of findings.
RESULTS
1722 publications in all were found during the first literature search. Following a meticulous assessment of abstracts and titles, 121 articles were deemed relevant, and their full texts were acquired for further examination. Excluded studies did not fulfill the inclusion criteria or did not explicitly investigate efficacy of wearable cardiac monitoring devices versus traditional methods in detecting atrial fibrillation. After a thorough screening procedure, ten papers were found to be appropriate for the systematic review and meta-analysis.
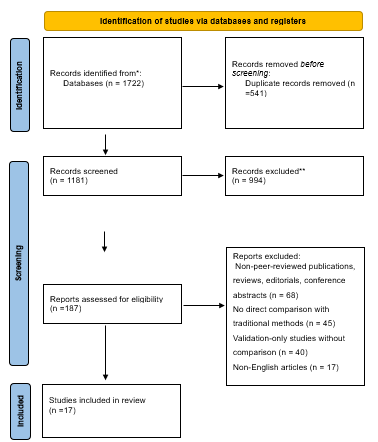
Figure 1. Prisma Flow Diagram
Study characteristics
The systematic review of wearable devices for atrial fibrillation (AF) detection included 17 studies. The bulk of the research (n = 10, 59 %) were prospective cohort studies, with a small number of cross-sectional observational studies (n = 3, 18 %). Case-control studies (n = 1, 6 %), randomized clinical trials (n = 1, 6 %), prospective pragmatic studies (n = 1, 6 %), siteless studies (n = 1, 6 %), and retrospective cohort assessments (n = 1, 6 %) made up a lesser percentage of the research. (table 1)
Patients recovering from heart surgery, older persons who had suffered a stroke, patients with TIA or cryptogenic stroke, patients with paroxysmal AF, and hospitalized participants were among the diverse demographics involved in the study. The number of participants varied across studies, with small cohorts (n = 15) and large cohorts (n = 419,297). The majority of research featured contrasts between wearable technology and conventional devices including ECG patches, Holter monitors, and implantable cardiac monitors (ICMs).
Numerous wearable devices were assessed during the trials, such as dyads of smartphone and wristwatch apps, adhesive ECG patches, patch-type ECG monitors, PPG-based mHealth devices, mobile telemetry, textile Holters, and standalone algorithms. While conventional equipment was usually used for continuous monitoring for 24 hours, wearable devices were utilized for monitoring for as little as 60 seconds and as long as 90 days.
Efficacy of Wearable Devices
Sensitivity and Specificity
The sensitivity and specificity of wearable devices to detect atrial fibrillation (AF) varied. For example, Wasserlauf et al. (2019) found that the Apple Watch with KardiaBand has a high sensitivity (97,5 %) for identifying bouts of AF. In a similar vein, Väliaho et al. (2019) discovered that a wristband PPG for AF detection had a 91,7 % sensitivity and a 98,1 % specificity. The Amazfit Health Band 1S was reported by Chen et al. (2020) to have specificities of 96,41 % and 99,20 %, respectively, with a sensitivity of 88,00 % (PPG) and 87,33 % (ECG). Furthermore, a wavelet PPG wristband was shown to be as accurate in identifying AF as the Alivecor KardiaBand ECG, with sensitivity and specificity of 100 % and 96 %, respectively, by Selder et al. (2020). According to this research, the accuracy of wearable technology—such as the Apple Watch, wristband PPG, and Amazfit Health Band 1S—often equals or surpasses that of conventional techniques, such Holter monitors and ECG patches.
Comparison of Wearable vs. Traditional Devices
When compared to conventional technologies, wearables often had detection capabilities that were either equal to or better. Hyun et al. (2024) discovered that while the MobiCARE-MC100 recognized an increasing AF load over a prolonged length of time, the patch-type ECG (MobiCARE-MC100) and 24-hour Holter ECG originally indicated AF in the same individuals. When compared to the 24-hour Holter ECG, Lang et al. (2021) showed that the Zio XT patch shortened the time needed for fitting and hospital visits. In contrast to wearable solutions, conventional devices such as 12-lead ECGs and insertable cardiac monitors (ICM) provided continuous monitoring but had drawbacks such greater dropout rates and false positives (Wouters et al., 2023; Rajakariar et al., 2023). (table 1)
Usability and Patient Compliance
Patient Preferences and Usability
Wearable technology is often thought to be very useful by people with chronic diseases and older persons who have had a stroke. According to Ding et al. (2022), 46 % of older persons who had had strokes thought the Samsung wristwatch and smartphone app pair was very useful. Although there were some difficulties, such as individuals who were already anxious or who were using assistance equipment, wearable technology was generally preferred over more conventional techniques. Additionally, Wouters et al. (2023) observed that mHealth and wristwatch technologies had better usability ratings because they were less invasive and more user-friendly. (table 1)
Compliance and Dropout Rates
The trials’ compliance rates varied, and several wearables had problems with prolonged usage. Due to signal quality problems, Dörr et al. (2019) reported a 21,8 % dropout rate for a wristwatch with PPG algorithm, while Pagola et al. (2021) found a 7 % dropout rate over 90 days for the textile Holter (Nuubo®). Notwithstanding these difficulties, wearable technology often outperformed conventional techniques in terms of compliance, particularly when it came to real-time feedback and ease of use. (table 1)
Extended Monitoring Benefits
Extended monitoring with wearable devices showed significant benefits in detecting AF. Hyun et al. (2024) reported that the MobiCARE-MC100 detected an increase in AF burden over 48 hours, which was not possible with the 24-hour Holter ECG.
Similarly, Lang et al. (2021) found that the Zio XT patch, with its 14-day monitoring period, detected AF cases more effectively than the 24-hour Holter ECG. Extended monitoring also proved beneficial in studies using the Apple Watch (Wasserlauf et al., 2019) and textile Holter (Pagola et al., 2021), with higher AF detection rates reported over longer periods.
Clinical Implications and Impact on Patient Care:
The use of wearable devices significantly impacted clinical pathways by reducing time to diagnosis and improving patient outcomes. Lang et al. (2021) demonstrated that the Zio XT patch facilitated quicker fitting and reduced hospital visits, streamlining the care pathway for stroke and TIA patients. Wasserlauf et al. (2019) found that the Apple Watch with KardiaBand provided a non-invasive and cost-effective approach for long-term AF surveillance, potentially reducing the need for more invasive procedures. Overall, wearable devices contributed to more efficient patient management, earlier detection of conditions, and improved long-term monitoring, thereby enhancing the overall quality of patient care. (table 1)
|
Table 1. Characteristics and results of the studies reviewed |
||||||||||
|
Author |
Year |
Population Type and Number |
Study Design |
Wearable Device |
Traditional Device |
Duration of Monitoring (Wearable & Traditional) |
Sensitivity |
Specificity |
Results with Stats |
Conclusion |
|
Seungji Hyun et al. (12) |
2024 |
Post-cardiac surgery patients, 39 |
prospective cohort study |
Patch-type ECG (MobiCARE-MC100) |
24-hour Holter ECG |
MobiCARE-MC100: 72 hours, Holter ECG: 24 hours |
Not specified |
Not specified |
Both methods identified A-fib in 7 out of 39 patients in the initial 24 hours. An increase in A-fib burden (9 %→38 %) was observed in 1 patient during the extended 48 hours. |
The MobiCARE device demonstrated comparable accuracy to Holter monitoring, and extended monitoring showed potential benefits for A-fib detection. |
|
Eric Y. Ding et al. (13) |
2022 |
Older adults’ post-stroke, 90 |
randomized clinical trial |
Smartwatch-smartphone app dyad (Samsung/Android) |
Patch monitor (Cardea SOLO™ ECG System) |
Samsung/Android: 14 days, Cardea SOLO™: 14 days |
Not specified |
Not specified |
46 % of participants found the smartwatch highly usable (SUS ≥68). Participants with assistive devices or anxiety reported higher anxiety with watch use. |
Older adults found the smartwatch usable and preferred it over traditional methods, but improvements in device interface and battery life are needed for better usability. |
|
Alex Lang et al. (14) |
2021 |
Cryptogenic stroke/TIA patients, 93 |
prospective cohort study |
Adhesive ECG patch (Zio XT) |
24-hour Holter ECG |
Zio XT: 14 days, Holter ECG: 24 hours |
Not specified |
Not specified |
The median time to fitting the monitor was significantly reduced with the Zio XT patch, and hospital visits were reduced by a median of two. |
The use of the Zio XT patch is feasible in routine clinical care, with a positive impact on patient care pathways by reducing time to fitting and hospital visits. |
|
Femke Wouters et al. (15) |
2023 |
Cryptogenic stroke/TIA patients, 39 |
Prospective cohort study |
PPG-based mHealth (smartphone/smartwatch) |
Insertable cardiac monitor (ICM) |
mHealth: 6 months, ICM: Continuous |
Not specified |
Not specified |
10 patients had AF detected by ICM, but only 1 confirmed. PPG-based mHealth also detected the confirmed AF. High false positives were observed in ICM readings. |
mHealth and ICM have potential for AF detection but require physician revision. mHealth compliance is a challenge without direct feedback, highlighting device limitations. |
|
Jorge Pagola et al. (16) |
2021 |
Cryptogenic stroke patients, 254 |
Prospective cohort |
Textile Holter (Nuubo®) |
traditional Holter monitoring |
Nuubo®: 90 days, N/A |
Not specified |
Not specified |
Cumulative incidence of AF detection at 90 days was 34,84 %. Monitoring time was similar across the 3 months. 7 % of patients abandoned monitoring. |
Intensive 90-day Holter monitoring with a textile Holter was feasible and detected a high percentage of AF. Enlarged left atrial volume predicted AF beyond the first week. |
|
Jonathan Krathen et al. (17) |
2021 |
Cryptogenic stroke patients, 15 |
Prospective cohort study |
Mobile telemetry (MT) |
Implantable loop recorders (ILR) |
MT: 4 weeks, ILR: Continuous |
Not specified |
Not specified |
AF was detected in 13 % of patients within the first 4 weeks on both MT and ILR. AF was detected in 1 patient at 92 days on the ILR. |
Early results showed MT was non-inferior to ILR in detecting early AF. Initial use of MT may be considered, reserving ILR for after an uneventful four-week period. |
|
Martin E. Matsumura et al. (18) |
2020 |
Cryptogenic stroke or TIA patients, 196 |
Retrospective cohort assessment |
Wearable ambulatory ECG (AECG) |
Inpatient Telemetry |
AECG: 14 days, N/A |
Not specified |
Not specified |
AF was detected in 12,8 % of patients. CHA2DS2Vasc ≤3 had a negative predictive value of 97,3 % for AF. Additional 5,3 % diagnosed with AF on long-term follow-up. |
Fourteen-day AECG diagnosed the majority of AF cases within 24 months following the event. CHA2DS2Vasc may help identify patients unlikely to show AF with 14-day AECG. |
|
Jeremiah Wasserlauf et al. (19) |
2019 |
Patients with paroxysmal AF, 24 |
Prospective cohort study |
Apple Watch with KardiaBand |
Insertable cardiac monitor (ICM) |
Apple Watch: Mean 11,3 hours/day, ICM: Continuous |
97,5 % |
Not specified |
The AFSW detected 80 out of 82 AF episodes ≥1 hour detected by the ICM. Episode sensitivity was 97,5 %, duration sensitivity was 97,7 %. |
The AFSW is highly sensitive for AF detection and assessment of AF duration when compared with an ICM. It represents an inexpensive, noninvasive approach for long-term AF surveillance. |
|
Rajakariar et al. (20) |
2020 |
200 patients |
Prospective cohort |
AliveCor KardiaBand (KB) |
12-lead ECG |
AliveCor KardiaBand: Immediate after iECG tracing, 12-lead ECG: Immediate after iECG tracing |
94,4 % |
81,9 % |
Moderate agreement between 12-lead ECG and KB (κ=0,60). PPV: 54,8 %; NPV: 98,4 %. EP interpretation improved accuracy (EP1: κ=0,76; EP2: κ=0,74). |
KB shows moderate accuracy; combining automated diagnosis with EP interpretation improves results. Physician involvement remains crucial. |
|
Dörr et al. (WATCH AF Trial) (21) |
2019 |
672 hospitalized subjects |
case-control |
Smartwatch with PPG algorithm |
Internet-enabled mobile ECG |
Not specified for both |
93,7 % |
98,2 % |
High accuracy (96,1 %). Dropout rate due to signal quality issues (21,8 %). |
Smartwatch detects AF with high accuracy, but signal quality issues limit applicability. Real-time checks needed for improvement. |
|
Ding et al. (22) |
2020 |
40 older adults |
prospective cohort study |
Smartwatch with novel algorithm |
Holter monitor |
Smartwatch: 42 minutes, Holter monitor: Simultaneous |
98,2 % |
98,1 % |
High accuracy (98,1 %) in identifying pulse irregularities. |
Algorithm shows high accuracy. Participants found the smartwatch highly acceptable despite age and comorbidities. |
|
Wasserlauf et al. (19) |
2018 |
24 patients |
Prospective cohort |
Apple Watch with KardiaBand |
Insertable cardiac monitor (ICM) |
Apple Watch: 11,3 (4,4) hours/day, ICM: 31,348,9 hours simultaneous monitoring |
97,5 % |
Not reported |
High sensitivity (97,5 %) for AF detection. PPV: 39,9 %. |
AFSW is highly sensitive for detecting AF and duration. Represents a cost-effective, noninvasive option for long-term AF monitoring. |
|
Perez et al. (23) |
2019 |
419,297 participants |
Prospective Pragmatic, siteless |
Apple Watch with iPhone app |
ECG patch |
Apple Watch: Median 117 days, ECG patch: Up to 7 days |
84 % |
Not reported |
34 % had AF on subsequent ECG patch readings. PPV for AF on ECG was 0,84. |
Low notification probability; 34 % with AF on ECG. Foundation for large-scale studies with user-owned devices. |
|
Väliaho et al. (24) |
2019 |
213 patients |
cross-sectional observational study |
Wristband PPG |
Electrocardiogram (ECG) |
Wristband PPG: 5 minutes, ECG: Simultaneous |
91,7 % (pulse) |
98,1 % (AF) |
PPG sensitivity: 91,7 %; PPV: 97,5 % for AF. Sensitivities for AF detection algorithms: 96,2 % and 95,3 %. |
PPG wristband enables accurate AF detection; algorithms show high accuracy. Suitable for AF screening. |
|
Selder et al. (25) |
2020 |
60 subjects (mean age 70±17) |
cross-sectional observational study |
Wavelet PPG wristband |
Alivecor KardiaBand |
Wavelet PPG wristband: 60 seconds, Alivecor KardiaBand: 30 seconds |
100 % |
96 % |
Sensitivity/specificity/PPV/NPV/accuracy: 100/96/75/100,97 % for PPG, 100/98/86/100/98 % for ECG. |
PPG wristband accurately detects AF, comparable to one-lead ECG. Suitable for long-term AF screening and monitoring. |
|
Rajakariar et al. (26) |
2019 |
200 patients (56,5 % male, age 67 ± 16) |
Prospective cohort study |
AliveCor KardiaBand |
12-lead ECG |
Not specified for both |
Not specified |
Not specified |
Manual clinician interpretation of unclassified traces concurred with 12-lead ECG in 89 % of cases. Strong inter-observer agreement (κ = 0,98, p < 0,001). |
High accuracy of clinician interpretation of unclassified iECGs. Efficient and cost-effective solution for arrhythmia screening. |
|
Chen et al. (27) |
2020 |
401 patients (251 normal, 150 AF) |
Cross- sectional Observational study |
Amazfit Health Band 1S |
Not specified |
Not specified for both |
88,00 % (PPG), 87,33 % (ECG) |
96,41 % (PPG), 99,20 % (ECG) |
Sensitivity/specificity/accuracy: 88,00 %/96,41 %/93,27 % for PPG; 87,33 %/99,20 %/94,76 % for ECG. Physician-judged ECG: 96,67 %/98,01 %/97,51 %. |
Combination of PPG, ECG, and AI algorithm facilitates AF detection. Potential for long-term monitoring. |
Quality assessment
The quality assessment of the cohort studies was conducted using the Newcastle-Ottawa Scale (NOS), which evaluates studies based on criteria such as the representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, and demonstration that the outcome of interest was not present at the start. The studies generally scored well across most categories, with several achieving maximum points in areas like comparability of cohorts, adequacy of follow-up, and assessment of outcomes.For example, studies by Alex Lang et al., Jeremiah Wasserlauf et al., and Rajakariar et al. received high scores across all categories, including representativeness of the exposed cohort, comparability of cohorts, and adequacy of follow-up. Studies by Jorge Pagola et al. and Jonathan Krathen et al., while generally strong, had some gaps, particularly in demonstrating that the outcome of interest was not present at the start and in the selection of non-exposed cohorts. (table 2)
|
Table 2. Quality assessment of the reviewed studies by New Castle Ottowa Scale |
|||||||||
|
Study |
Representativeness of the exposed cohort (1) |
Selection of the non-exposed cohort(1) |
Ascertainment of exposure (1) |
Demonstration that outcome of interest was not present at start of study(1) |
Compare ability of cohorts on the basis of the design or analysis (2) |
Assessment of outcome (1) |
Was follow-up long enough for outcomes to occur(1) |
Adequacy of follow up of cohorts (1) |
Representativeness of the exposed cohort (1) |
|
Seungji Hyun et al. (12) |
1 |
1 |
1 |
|
2 |
1 |
1 |
1 |
1 |
|
Alex Lang et al. (14) |
1 |
1 |
1 |
1 |
2 |
1 |
1 |
1 |
1 |
|
Femke Wouters et al. (15) |
1 |
1 |
1 |
|
2 |
1 |
1 |
1 |
1 |
|
Jorge Pagola et al. (16) |
1 |
|
1 |
|
1 |
1 |
1 |
1 |
1 |
|
Jonathan Krathen et al. (17) |
1 |
|
1 |
|
1 |
1 |
1 |
1 |
1 |
|
Martin E. Matsumura et al. (18) |
1 |
1 |
1 |
|
2 |
1 |
1 |
1 |
1 |
|
Jeremiah Wasserlauf et al. (19) |
1 |
1 |
1 |
1 |
2 |
1 |
1 |
1 |
1 |
|
Rajakariar et al. (20) |
1 |
1 |
1 |
|
2 |
1 |
1 |
1 |
1 |
|
Ding et al. (22) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Wasserlauf et al. (19) |
1 |
|
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Rajakariar et al. (26) |
1 |
1 |
1 |
1 |
2 |
1 |
1 |
1 |
1 |
Meta-analysis
There is a significant amount of variation in the sensitivity of wearable devices for detecting atrial fibrillation, according to a pooled analysis of 9 studies. Assuming homogeneity across studies, the fixed-effect model estimates an overall sensitivity of 91,59 % with a narrow confidence interval (95 % CI: 89,22 % to 93,48 %), indicating high sensitivity. The heterogeneity-accounting random-effects model provides a slightly higher estimate of 94,03 % (95 % CI: 90,05 % to 96,49 %). A tau² of 0,4480, an I² value of 70,2 %, and an H value of 1,83 (95 % CI: 1,30 to 2,58) suggest considerable variance beyond sampling error, indicating substantial heterogeneity among the studies. The test of heterogeneity (Q = 26,88, p = 0,0007) further supports this significant discrepancy.

Figure 2. Forest plot regarding the sensitivity of the wearable devices
There is a significant amount of variation in the specificity of wearable devices for detecting atrial fibrillation (AF), according to a pooled analysis of 6 studies. Assuming homogeneity across studies, the fixed-effect model estimates an overall specificity of 92,13 % with a narrow confidence interval (95 % CI: 89,01 % to 94,42 %), indicating high specificity. The heterogeneity-accounting random-effects model provides a slightly higher estimate of 95,96 % (95 % CI: 89,63 % to 98,49 %). A tau² of 1,2596, an I² value of 82,9 %, and an H value of 2,41 (95 % CI: 1,66 to 3,51) suggest considerable variance beyond sampling error, indicating substantial heterogeneity among the studies. The test of heterogeneity (Q = 29,16, p < 0,0001) further supports this significant discrepancy.

Figure 2. Forest plot regarding the specificity of the wearable devices
DISCUSSION
This review and meta-analysis reveal that wearable cardiac monitoring devices show considerable variation in their efficacy compared to traditional methods for detecting atrial fibrillation. The pooled analysis from various studies demonstrates that wearable devices generally possess high sensitivity and specificity, although significant variability exists across different devices and study designs.
Overall, wearable devices exhibit varying degrees of sensitivity and specificity in AF detection. For instance, devices like the Apple Watch with KardiaBand and the AliveCor KardiaBand show high sensitivity for AF detection, closely matching or even surpassing traditional methods such as Holter monitors and ILRs in some cases.(19,20) Devices such as the Apple Watch with KardiaBand and the Wavelet PPG Wristband achieved particularly high sensitivity, with figures of 97,5 % and 100 %, respectively.(19,25) The high sensitivity indicates that these wearable devices are effective at identifying episodes of AF, which is crucial for early detection and management. This is consistent with previous studies of literature, such as a systematic review of 208 studies on the use of mobile health solutions for the detection of AF, which found that the sensitivity and specificity of ECG-based devices were as follows: The percentages for belts, patches, portable devices, and belt-mounted devices are 95,6 %–98,5 %, 76,0 %–95,0 %, and 93,4 %–97,0 %, respectively.(28) High sensitivity (94 %, 95 % CI 92-25) and specificity (97 %, 95 % CI 96-98) were seen for AF identification in a meta-analysis of 28 trials comparing smartphone PPG against ECG for AF detection.(29) Nevertheless, there was significant inter-study variability in the low-quality studies, which also carried a high risk of selection and publication bias.
Conversely, the specificity of these wearable devices varies. For example, wearable devices like the PPG-based mHealth system and the Zio XT patch demonstrate a high detection rate but also exhibit challenges such as high false positives.(14,15) However, some devices, such as the insertable cardiac monitor (ICM), exhibited lower specificity due to higher false-positive rates.(15) This discrepancy underscores the importance of combining wearable technology with physician oversight to minimize false positives and ensure accurate diagnosis. The Amazfit Health Band 1S, for example, showed high specificity of 99,20 % for ECG readings.(27)
When comparing wearable devices to traditional methods like Holter monitors and 12-lead ECGs, several important observations emerge. Wearable devices offer the advantage of extended monitoring durations compared to traditional methods. For instance, the Nuubo® textile Holter provided continuous monitoring for up to 90 days, leading to a 34,84 % cumulative incidence of AF detection.(16) This extended monitoring can enhance AF detection rates compared to the typical 24-hour duration of traditional Holter monitors.(12,14) Thirty-day smartphone ECG monitoring with KardiaMobile (AliveCor) revealed an absolute difference of 7,5 % in AF detection (P = 0,024) compared to 24-hour Holter monitoring, according to a multicenter, open-label RCT on patients aged ≥55 having ischemic stroke or TIA in the previous 12 months.(30)
Wearable technology adoption has been increasing year on year, and is estimated to reach more than 1 billion users in 2022, which may increase the acceptability and cost-effectiveness of wearable devices for cardiac monitoring of patients. Usability and patient preference also play significant roles. Wearable devices often present improved usability and higher patient acceptance. The smartwatch with a novel algorithm was preferred by older adults over traditional patch monitors, despite issues related to interface and battery life.(22) The high usability of devices like the Apple Watch further supports their potential for better patient adherence to long-term monitoring.(19)
While wearable devices show high accuracy, they are not always equivalent to traditional methods. For instance, the AliveCor KardiaBand demonstrated moderate agreement with 12-lead ECGs, indicating that while wearable devices can be highly effective, they may not completely replace traditional diagnostic methods.(20) Additionally, mobile telemetry (MT) has been found to be non-inferior to implantable loop recorders (ILR) for early AF detection, suggesting that wearable devices can be a viable alternative in specific scenarios.(17)
The meta-analysis revealed substantial heterogeneity among the included studies. For sensitivity, the fixed-effect model estimated an overall proportion of 91,59 % (95 % CI: 89,22 % to 93,48 %), while the random-effects model provided an estimate of 94,03 % (95 % CI: 90,05 % to 96,49 %). The heterogeneity measures (I² = 70,2 %) indicate significant variance beyond sampling error. For specificity, the fixed-effect model estimated a proportion of 92,13 % (95 % CI: 89,01 % to 94,42 %), and the random-effects model estimated 95,96 % (95 % CI: 89,63 % to 98,49 %), with an I² value of 82,9 %. These findings suggest substantial variability in study outcomes, necessitating caution in interpreting pooled results.
CONCLUSIONS
Wearable cardiac monitoring devices have demonstrated considerable potential in detecting AF with high sensitivity and specificity. However, their performance varies widely, and they may not yet completely replace traditional methods like Holter monitors or 12-lead ECGs. The review highlights the benefits of extended monitoring durations and improved patient compliance associated with wearable devices. Still, it also points out the need for continued advancements in device accuracy, usability, and integration into clinical workflows. Future research should focus on refining algorithms, reducing false-positive rates, and exploring the most effective use cases for wearable devices in AF detection.
REFERENCES
1. Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14(3):195. doi:10.11909/J.ISSN.1671-5411.2017.03.011
2. Majos E, Dabrowski R. Significance and Management Strategies for Patients with Asymptomatic Atrial Fibrillation. J Atr Fibrillation. 2015;7(5):1169. doi:10.4022/JAFIB.1169
3. Lin HJ, Wolf PA, Benjamin EJ, Belanger AJ, D’Agostino RB. Newly Diagnosed Atrial Fibrillation and Acute Stroke. Stroke. 1995;26(9):1527-1530. doi:10.1161/01.STR.26.9.1527
4. Lewis M, Parker D, Weston C, Bowes M. Screening for atrial fibrillation: sensitivity and specificity of a new methodology. Br J Gen Pract. 2011;61(582):38-39. doi:10.3399/BJGP11X548956
5. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. doi:10.1016/J.JACC.2014.03.022
6. Curry SJ, Krist AH, Owens DK, et al. Screening for Atrial Fibrillation With Electrocardiography: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(5):478-484. doi:10.1001/JAMA.2018.10321
7. Mandrola J, Health Louisville B, Andrew Foy K. Downsides of Detecting Atrial Fibrillation in Asymptomatic Patients. Am Fam Physician. 2019;99(6):354-355. Accessed July 20, 2024. https://www.aafp.org/pubs/afp/issues/2019/0315/p354.html
8. Turakhia MP, Kaiser DW. Transforming the care of atrial fibrillation with mobile health. J Interv Card Electrophysiol. 2016;47(1):45-50. doi:10.1007/S10840-016-0136-3/METRICS
9. Nazarian S, Lam K, Darzi A, Ashrafian H. Diagnostic accuracy of smartwatches for the detection of cardiac arrhythmia: Systematic review and meta-analysis. J Med Internet Res. 2021;23(8):e28974. doi:10.2196/28974
10. Ding EY, Marcus GM, McManus DD. Emerging Technologies for Identifying Atrial Fibrillation. Circ Res. 2020;127(1):128-142. doi:10.1161/CIRCRESAHA.119.316342/ASSET/1525E325-B34E-4441-8337-78B65B16FE6A/ASSETS/IMAGES/LARGE/CIRCRESAHA.119.316342.FIG03.JPG
11. Belani S, Wahood W, Hardigan P, Placzek AN, Ely S. Accuracy of Detecting Atrial Fibrillation: A Systematic Review and Meta-Analysis of Wrist-Worn Wearable Technology. Cureus. 2021;13(12). doi:10.7759/CUREUS.20362
12. Hyun S, Lee S, Hong YS, Lim S hyun, Kim DJ. Evaluation of the Diagnostic Performance and Efficacy of Wearable Electrocardiogram Monitoring for Arrhythmia Detection after Cardiac Surgery. J Chest Surg. 2024;57(2):205. doi:10.5090/JCS.23.152
13. Ding EY, CastañedaAvila M, Tran K Van, et al. Usability of a smartwatch for atrial fibrillation detection in older adults after stroke. Cardiovasc Digit Heal J. 2022;3(3):126-135. doi:10.1016/J.CVDHJ.2022.03.003
14. Lang A, Basyal C, Benger M, et al. Process and Systems: Improving stroke pathways using an adhesive ambulatory ECG patch: reducing time for patients to ECGs and subsequent results. Futur Healthc J. 2022;9(1):64-66. doi:10.7861/FHJ.2021-0151
15. Wouters F, Gruwez H, Vranken J, et al. The Potential and Limitations of Mobile Health and Insertable Cardiac Monitors in the Detection of Atrial Fibrillation in Cryptogenic Stroke Patients: Preliminary Results From the REMOTE Trial. Front Cardiovasc Med. 2022;9:848914. doi:10.3389/FCVM.2022.848914/BIBTEX
16. Pagola J, Juega J, Francisco J, et al. Abstract P624: High Detection of Atrial Fibrillation by 90 Days Textil Holter Monitoring in Patients With Cryptogenic Stroke. Stroke. 2021;52(Suppl_1). doi:10.1161/STR.52.SUPPL_1.P624
17. Krathen J, Tan JL, Essilfie G, Ortman M, Andriulli J, Russo A. SHOULD MOBILE TELEMETRY MONITORING OR IMPLANTABLE LOOP RECORDERS BE UTILIZED FOR EARLY DETECTION OF SILENT ATRIAL ARRHYTHMIAS IN PATIENTS WITH CRYPTOGENIC STROKE? J Am Coll Cardiol. 2021;77(18):415. doi:10.1016/S0735-1097(21)01774-5
18. Matsumura ME, Amin H, Khalil Y, Subzposh F, Martin B. UTILITY OF 14 DAY AMBULATORY ECG IN THE DIAGNOSIS OF ATRIAL FIBRILLATION FOLLOWING CRYPTOGENIC STROKE. J Am Coll Cardiol. 2020;75(11):415. doi:10.1016/S0735-1097(20)31042-1
19. Wasserlauf J, You C, Patel R, Valys A, Albert D, Passman R. Smartwatch Performance for the Detection and Quantification of Atrial Fibrillation. Circ Arrhythmia Electrophysiol. 2019;12(6). doi:10.1161/CIRCEP.118.006834/SUPPL_FILE/CIRCAE_CIRCAE-2018-006834_SUPP1.PDF
20. Rajakariar K, Koshy AN, Sajeev JK, Nair S, Roberts L, Teh AW. Accuracy of a smartwatch based single-lead electrocardiogram device in detection of atrial fibrillation. Heart. 2020;106(9):665-670. doi:10.1136/HEARTJNL-2019-316004
21. Dörr M, Nohturfft V, Brasier N, et al. The WATCH AF Trial: SmartWATCHes for Detection of Atrial Fibrillation. JACC Clin Electrophysiol. 2019;5(2):199-208. doi:10.1016/J.JACEP.2018.10.006
22. Ding EY, Han D, Whitcomb C, et al. Accuracy and Usability of a Novel Algorithm for Detection of Irregular Pulse Using a Smartwatch Among Older Adults: Observational Study. JMIR cardio. 2019;3(1):e13850. doi:10.2196/13850
23. Perez M V., Mahaffey KW, Hedlin H, et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381(20):1909-1917. doi:10.1056/NEJMOA1901183/SUPPL_FILE/NEJMOA1901183_DATA-SHARING.PDF
24. Väliaho ES, Kuoppa P, Lipponen JA, et al. Wrist band photoplethysmography in detection of individual pulses in atrial fibrillation and algorithm-based detection of atrial fibrillation. EP Eur. 2019;21(7):1031-1038. doi:10.1093/EUROPACE/EUZ060
25. Selder JL, Proesmans T, Breukel L, et al. Assessment of a standalone photoplethysmography (PPG) algorithm for detection of atrial fibrillation on wristband-derived data. Comput Methods Programs Biomed. 2020;197:105753. doi:10.1016/J.CMPB.2020.105753
26. Rajakariar K, Koshy A, Sajeev J, Nair S, Roberts L, Teh A. Smartwatch Based Arrhythmia Detection: Accuracy of Clinician Interpretation of Unclassified Tracings. Hear Lung Circ. 2019;28:S228. doi:10.1016/j.hlc.2019.06.221
27. Chen E, Jiang J, Su R, et al. A new smart wristband equipped with an artificial intelligence algorithm to detect atrial fibrillation. Hear Rhythm. 2020;17(5):847-853. doi:10.1016/j.hrthm.2020.01.034
28. Hermans ANL, Gawalko M, Dohmen L, et al. Mobile health solutions for atrial fibrillation detection and management: a systematic review. Clin Res Cardiol. 2022;111(5):479-491. doi:10.1007/S00392-021-01941-9/FIGURES/3
29. Gill S, Bunting K V., Sartini C, et al. Smartphone detection of atrial fibrillation using photoplethysmography: a systematic review and meta-analysis. Heart. 2022;108(20):1600-1607. doi:10.1136/HEARTJNL-2021-320417
30. Koh KT, Law WC, Zaw WM, et al. Smartphone electrocardiogram for detecting atrial fibrillation after a cerebral ischaemic event: a multicenter randomized controlled trial. EP Eur. 2021;23(7):1016-1023. doi:10.1093/EUROPACE/EUAB036
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: Galo Fernando Tulcanaza Ochoa, Javier Lizandro Meza Tonato, Paola Gissela Placencia Guartatanga.
Data Curation: Paulina Elizabeth Cisneros Clavijo, Mónica Paulina Manzano Vela, Adriana Viviana Viñan Andino.
Formal Analysis: Javier Lizandro Meza Tonato, Néstor Raúl Parrales Ponce, Franklin Isaac Nieto Nuñez.
Research: Paola Gissela Placencia Guartatanga, Adriana Viviana Viñan Andino, Paulina Elizabeth Cisneros Clavijo.
Methodology: Néstor Raúl Parrales Ponce, Galo Fernando Tulcanaza Ochoa, Paola Gissela Placencia Guartatanga.
Project Management: Mónica Paulina Manzano Vela, Javier Lizandro Meza Tonato, Franklin Isaac Nieto Nuñez.
Software: Galo Fernando Tulcanaza Ochoa, Franklin Isaac Nieto Nuñez, Néstor Raúl Parrales Ponce.
Supervision: Javier Lizandro Meza Tonato, Mónica Paulina Manzano Vela, Galo Fernando Tulcanaza Ochoa.
Validation: Paulina Elizabeth Cisneros Clavijo, Adriana Viviana Viñan Andino, Néstor Raúl Parrales Ponce.
Visualization: Franklin Isaac Nieto Nuñez, Mónica Paulina Manzano Vela, Paulina Elizabeth Cisneros Clavijo.
Drafting - Original Draft: Mónica Paulina Manzano Vela, Paola Gissela Placencia Guartatanga, Adriana Viviana Viñan Andino.
Writing - Proofreading and Editing: Adriana Viviana Viñan Andino, Javier Lizandro Meza Tonato, Paulina Elizabeth Cisneros Clavijo.