doi: 10.56294/saludcyt2024.920
ORIGINAL
Evaluate the Frequency of Eosinophilic Esophagitis in Individuals with Refractory GERD
Evaluar la frecuencia de la esofagitis eosinofílica en individuos con ERGE refractaria
Nithesh Babu Ramesh1 ![]() *,
Prasanna Venkatesh2
*,
Prasanna Venkatesh2 ![]() *,
Tusha3
*,
Tusha3 ![]() *,
Neha Nanditha Adepu4
*,
Neha Nanditha Adepu4 ![]() *,
Abinaya Srinivasa Rangan5
*,
Abinaya Srinivasa Rangan5
![]() *,
Shubhansh Bansal6
*,
Shubhansh Bansal6 ![]() *
*
1Saveetha Institute of Medical and Technical Sciences, Saveetha University, Department of General Medicine. Chennai, India.
2Mahatma Gandhi Medical College and Research Institute. Sri Balaji vidyapeeth (deemed to be university), Department of General Medicine. Pondicherry, India.
3Quantum University Research Center. Uttarakhand, India.
4Osmania Medical College, Department of MBBS. Telangana, India.
5Saveetha Institute of Medical and Technical Sciences, Saveetha University, Department of General Medicin. Chennai, India.
6Chitkara University, Department of Research Impact and Outcome. Punjab, India.
Cite as: Babu Ramesh N, venkatesh P, Tusha T, Adepu NN, Srinivasa Rangan A, Bansal S. Evaluate the Frequency of Eosinophilic Esophagitis in Individuals with Refractory GERD. Salud, Ciencia y Tecnología. 2024; 4:.920. https://doi.org/10.56294/saludcyt2024.920
Submitted: 24-12-2024 Revised: 17-04-2024 Accepted: 23-08-2024 Published: 24-08-2024
Editor: Dr.
William Castillo-González ![]()
ABSTRACT
Introduction: the eosinophilic infiltrate of the esophagus mucosal distinguishes the esophageal ailment known as Eosinophilic esophagitis (EoE).
Objective: to determine the frequency of EoE in patients with gastroesophageal reflux disease (GERD).
Method: the 248 children in total had elective upper gastrointestinal endoscopy (UGIE) during the study period were separated in tosix groups: individuals without a history of steroid usage and those without an esophageal biopsy for thrombocytopenia. As a result, 200 people participated in the study total.
Results: after an evaluation of 200 children having elective UGIE, A variety of health problems were found during the investigation, including food bolus impact (2 %), achalasia cardia (5 %), peptic stricture (6 %), congenital stricture (3 %), post-esophageal atresia repair anastomotic stricture (4 %), and symptoms of gastro esophageal reflux, including vomiting (10 %), regurgitation (2 %), belching chest (1,5 %), nocturnal cough (2,5 %), pain/heartburn (12,5 %), and feeding refusal (1 %). There were several unusual illnesses that were observed, including Crohn’s disease accounted for 2 %, pediatric intestinal pseudo-obstruction (10 %), abdominal discomfort (17,5 %), celiac disease (16 %), cow’s milk protein allergy (3 %), and un classified IBD (7 %).
Conclusion: EoE frequently affects GERD patients who are recalcitrant to treatment. Dysphagia, food impaction, and heartburn are possible symptoms. EGD and esophageal biopsy should be explored for EoE screening in elderly individuals with strong GERD who are atopy positive and who have not responded to gastric acid inhibitors.
Keywords: EoE; Refractory GERD; Frequent Endoscopic; Elder Patients.
RESUMEN
Introducción: el infiltrado eosinofílico de la mucosa esofágica distingue la dolencia esofágica conocida como esofagitis eosinofílica (EoE).
Objetivo: determinar la frecuencia de la EoE en pacientes con enfermedad por reflujo gastroesofágico (ERGE), una afección gastroesofágica persistente.
Método: los 248 niños en total a los que se les realizó endoscopia gastrointestinal superior electiva (EoE) durante el periodo de estudio fueron separados en dos grupos: individuos sin antecedentes de uso de esteroides y aquellos con biopsia esofágica por trombocitopenia. En total, participaron en el estudio 200 personas.
Resultados: tras una evaluación de 200 niños sometidos a UGIE electiva, se encontraron diversos problemas de salud durante la investigación, como impacto de bolo alimenticio (2 %), cardias acalasia (5 %), estenosis péptica (6 %), estenosis congénita (3 %), post reparación de atresia de la anastomosis esofágica (4 %), y síntomas de reflujo gastroesofágico, incluidos vómitos (10 %), regurgitación (2 %), eructos en el pecho (1,5 %), tos nocturna (2,5 %), dolor/acidez (12,5 %) y rechazo de la alimentación (1 %). Se observaron varias enfermedades inusuales, como la enfermedad de Crohn (2 %), pseudo obstrucción intestinal pediátrica (10 %), molestias abdominales (17,5 %), celiaquía (16 %), alergia a las proteínas de la leche de vaca (3 %) y EII no clasificada (7 %).
Conclusiones: la EoE afecta con frecuencia a pacientes con ERGE recalcitrantes al tratamiento. La disfagia, la impactación de alimentos y la pirosis son síntomas posibles. La EGD y la biopsia esofágica deberían explorarse para el cribado de la EoE en ancianos con ERGE intensa que presentan atopia positiva y que no han respondido a los inhibidores de la acidez gástrica.
Palabras clave: EoE; ERGE Refractaria; Endoscopia Frecuente; Pacientes Ancianos.
INTRODUCTION
Millions of people across the world suffer from the ailment known as GERD. It is stomach acid or bile runs back into the esophagus, resulting in symptoms including heartburn, regurgitation, and pain in the chest. Acid-suppressing medications treat the majority of GERD patients, and few patients may develop refractory GERD, which results in symptoms that worsen even after obtaining the most effective practical medical therapy.(1) White blood cells called eosinophils are the characteristic of eosinophil esophagitis (EoE), a chronic esophageal inflammatory illness that mimics GERD in symptoms such as heartburn, chest pain, and difficulty swallowing.(2,3) This is a lower frequency than previously reported among of differences in patient groups and diagnostic criteria. Using esophageal samples obtained through a higher endoscopy, research evaluated the prevalence of EoE in individuals with rejected GERD.(4)
Due to potential overlap in symptoms and endoscopic results, and the possibility that some individuals are show normal endoscopic findings, diagnosing EoE and uncontrolled GERD can be challenging. To prove the diagnosis, it’s advised that individuals with difficult GERD include EoE undergo an esophageal biopsy.(5) To determine the most appropriate line of action, clinicians should confirm cases of persistent GERD and EoE with an esophageal biopsy and comprehend the connection among these environment.(6) Following its first classification in children, EoE instance in adults have now recently been documented; this increase in cases gets credited to changes in genetics, ecological variables, and more comprehensive search for the illness.(7,8) Despite acid suppression, esophagitis is common in people with esophageal dysmotility; this is probably due to inadequate esophageal clearance.
The dysmotility condition esophageal atresia (EA) is caused by changes in CYP2C19 metabolism, which in non-dysmotility populations leads to Proton pump inhibitor (PPI) resistance and non-allergic esophagitis.(9) Background Global awareness of EoE is rising, although information on the condition in India is limited. The study looked at GERD symptoms in individuals that had esophageal biopsies, gastroduodenoscopies, or any other endoscopically evident aberrant mucosa. The upper esophagus was sampled and the lower esophagus was sampled.(10) EoE is more common among kids with esophageal atresia, according to recent investigations. Because of untreated chronic inflammation, people with EoE may abuse antireflux medications and need more surgeries, which can result in excessive fundoplication usage and recurrent strictures.(11) The elimination diet is the sole therapy for EoE, a chronic inflammatory esophageal illness that is predominantly brought on by food antigens. Importantly, EoE was a distinct type of delayed, cell-mediated hypersensitivity and a non-IgE-mediated food allergy.(12) A number of variables, including cytokines, acid reflux, eosinophilic esophagitis, and disruption of the tight junction (TJ) protein, might affect the symptoms of GERD, especially heartburn.(13) Approximately one-third of patients might have a resistant type of EoE, which is defined by non-responsiveness in clinical, endoscopic, or histological evaluation following first-line treatment.(14) Due to the symptoms and histologic similarities between EoE and GERD, diagnosing EoE can be difficult.(15) EoE is a clinicopathological disorder mediated by the immune system that is characterized by esophageal eosinophil infiltration leading to chronic inflammation and stricture.(16) The research included medical, endoscopic, and pH-impedance testing to assess risk factors for esophageal stricture and EoE in children with esophageal atresia and concomitant EoE. This cohort was then compared to disease-matched controls.(17) Both children and adults can develop the chronic immunological disorder EoE.
To confirm a diagnosis of EoE, a biopsy is performed after an endoscopic assessment for dysphagia. There’s also a chance of reflux-like symptoms.(18) The clinical demonstration of esophageal dysfunction in EoE, an constant provocative provision of the esophagus, is esophageal dysfunction.(19) One of a number of gastrointestinal situations known as eosinophilic disorders, whose inflammation has no secondary causes, is EoE.(20) The objective of the study was to ascertain if aberrations in high-resolution manometry motility are associated with eosinophil-induced esophageal inflammation, and whether this influences immune-mediated provision.(21) The purpose of the study was to identify topographic patterns of esophageal motility in EoE (epithelial inflammation), an immune-mediated inflammation that predominantly affects the esophagus, and to ascertain if symptoms correspond with motility abnormalities shown on HRM.(22) An estimated 34,4/100 000 people in suffer from EoE, a chronic immune-related inflammatory poor health of the esophagus. Dysphagia, esophageal strictures, and food impaction are among the symptoms of EoE that can entail both children and adults.(23) Esophageal dysphagia and inflammation, predominantly mediated by eosinophils, are the outcomes of T-helper type 2-induced chronic autoimmune esophageal illness (EoE).(24) The incidence of EoE is rising even though the only diagnostic indicators available were esophageal symptoms and eosinophilia on biopsies (25).
METHODS
Participants
The 248 children in all received elective UGIE throughout the research period; of these, 20 have a history of using steroids and were therefore excluded, and 28 were not given an esophageal biopsy for thrombocytopenia. Thus, the study eventually comprised 200 participants.
Statistical analysis
The incidence of EoE in obstinate GERD patients is approximated at 10 % using the proportional technique due to a paucity of published data. For statistical significance, 138 patients were needed based on prevalence. We chose to recruit 150 patients to replace the projected 10 % dropout rate.
For categorical data, medians and ranges were used for Chi2-test analysis. To test contingency table cells with expected values below 5, Fisher’s exact test is utilized. Percentage of EoE is calculated using a 95 % confidence interval (95 % CI). The U-test is used to examine the means and standard deviations of continuous information. To find independent predictors of EoE, logistic regression analysis is performed on relevant factors from the univariate study. Probability values below 0,05 were deemed significant, for our statistical analysis, SPSS Version 16,0 is utilized.
RESULTS
After 200 children had elective UGIE, the results of the investigation showed that individuals had a variety of illnesses and disease symptoms. Food bolus impaction (1 %) achalasia cardia (2,5 %), peptic stricture (3 %), congenital stricture (1,5 %), and post-esophageal atresia repair anastomotic stricture (2 %), among other disorders, is associated with dysphagia. Thesymptomsassociatedwithgastroesophagealrefluxdiseaseincludedvomiting (10 %), regurgitation (2 %), belching chest (1,5 %), nocturnal cough (2.5 %), pain/heartburn (12.5 %), and a refusal to feed (1 %). The Crohn’s disease (2 %) and other inflammatory diseases were identified. In addition to pediatric intestinal pseudo-obstruction (10 %), evaluation of abdominal discomforts numerous cases (17,5 %) without precise classification. Celiac disease (16 %), cow’s milk protein allergy (3 %), and unclassified IBD (7 %) were among the bowel illnesses. Rumination syndrome, cyclic vomiting syndrome, systemic lupus erythematosus, juvenile idiopathic arthritis, and primary intestinal lymphangiectasia is among the other unusual illnesses that were noted. Table 1 shows the main causes of elective upper gastrointestinal surgery. Figure 1 shows the Gastroesophageal reflux-like symptoms. Figure 2 shows the outcome of Dysphagia.
|
Table 1. Main causes of elective upper gastrointestinal surgery |
||
|
Disease / symptoms |
Infections |
N (%) |
|
Dysphagia |
Food bolus impaction |
2 (1) |
|
Achalasia cardia |
5 (2,5) |
|
|
Peptic stricture |
6 (3) |
|
|
Congenital stricture |
3 (1,5) |
|
|
Post-esophageal atresia repair anastomotic stricture |
4 (2) |
|
|
Gastroesophageal symptoms |
Vomiting |
20 (10) |
|
Nocturnal cough |
5 (2,5) |
|
|
Belching Chest |
3 (1,5) |
|
|
Pain/heartburn |
25 (12,5) |
|
|
Regurgitation |
4 (2) |
|
|
Refusal to feed |
2 (1) |
|
|
Inflammatory |
Crohn’s disease |
4 (2) |
|
Evaluation of abdominal pain |
- |
35 (17,5) |
|
Pediatric intestinal pseudo obstruction - evaluation of abdominal pain |
- |
20 (10) |
|
Bowel disease |
IBD not categorized |
14 (7) |
|
Celiac disease |
- |
32 (16) |
|
Cow’s milk protein allergy |
- |
6 (3) |
|
Others |
Overall |
10 (5) |
|
Rumination syndrome |
3 |
|
|
Cyclic vomiting syndrome |
2 |
|
|
Juvenile idiopathic arthritis |
2 |
|
|
Systemic lupus erythematosus |
2 |
|
|
Primary intestinal lymphangiectasia |
1 |
|
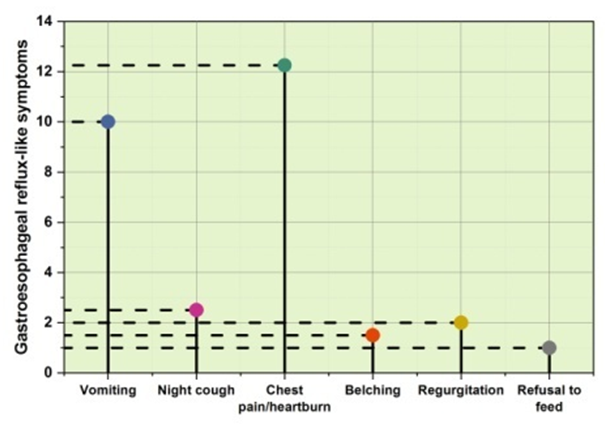
Figure 1. Gastroesophageal reflux-like symptoms

Figure 2. Outcome of Dysphagia
DISCUSSION
In terms of the frequency of EoE in resistant GERD patients, their investigation is the first. Retrospective study raises the possibility of memory bias. Patients reported having an incidence of EoE during higher gastrointestinal endoscopy for any cause. The patients were younger and more male-dominated. All patients exhibited feline esophageal features, according to an endoscopy, it also revealed food impaction, indicators of dysphagia, and a history of atopy, some of which contained micro-abscesses. Chronic acid exposure in GERD damages and inflames the esophageal mucosa, which causes moderate eosinophilia. Given the high incidence of GERD in all individuals, there is conceivable for EoE and GERD to coexist given randomness. The findings of pH tests on EoE patients support the theory that EoE may arise after a protracted GERD episode by demonstrating chronic acid exposures. This theory is supported by the evidence that eosinophils cause remodeling by rupturing the mucosal barrier and esophageal smooth muscles. The lower esophageal sphincter becomes slack as a result of the remodeling impact, which also affects acid clearance and causes GERD symptoms.
CONCLUSION
EoE frequently develops in GERD patients. The majority of these patients are elderly women. Food impaction, heartburn, and dysphagia are prevalent in these patients. Friability, Strictures are also common endoscopic findings, along with white plaques and erosive esophagitis. GERD, atopy, and esophageal biopsies should be investigated for the EoE screening of older patients who are not responding to gastric acid inhibitors. Frequent eosinophil infiltration (Frequent EoE) is a long-term immune-mediated esophageal disease that is frequently brought on by resistant GERD. Even though GERD is a common condition, some people may continue to have symptoms even after following suggested treatment, a condition known as refractory GERD. According to research, the actual occurrence of EoE in persons with unresponsive GERD has been reported. EoE is considerably more ordinary in people with refractory GERD, however, recommend that clinicians should take esophagus biopsies to rule out EoE in patients who still feel symptoms despite being paid effective GERD handling. Due to the common misdiagnosis of EoE as GERD and the danger of consequences including esophageal strictures and dysphagia from delayed diagnosis and treatment of EoE, this is especially crucial. To fully comprehend how EoE and refractory GERD are related, more study is required. Consensus recommendations are also necessary for the organization of patients with refractory GERD and supposed EoE, including the right diagnostic measures and accessible therapies. Patients with chronic GERD may benefit from esophageal biopsies to rule out EoE, as postponing diagnosis and treatment might have unfavorable effects.
REFERENCES
1. Carlos, Gustavo Campanha Menon, et al. “Treatment for Eosinophilic Esophagitis in adults: Where are we?.”Seven Editora (2023). doi:10.56238/devopinterscie-150
2. Patel, Ankit D., et al., eds. The SAGES Manual of PhysiologicEvaluation of ForegutDiseases. Springer, 2023.
3. Ridolo, Erminia, et al. “Assessing the risk factors for refractory eosinophilic esophagitis in children and adults.” GastroenterologyResearch and Practice 2019.1 (2019): 1654543.doi:10.1155/2019/1654543
4. Wilson, Bridget E., et al. “The Relationship Between Eosinophilic Esophagitis and Immunotherapy.” Immunology and AllergyClinics (2024). doi:10.1016/j.iac.2024.01.001
5. Zarzycka, Marta, et al. “Understanding of EosinophilicEsophagitis in Children-A ComprehensiveStudyonEpidemiology, ClinicalManifestations, Diagnosis and InnovativeTreatmentModalities.” Journal of Education, Health and Sport 55 (2024): 137-154. doi:10.12775/JEHS.2024.55.009
6. Patel, Amit, and RenaYadlapati. “Diagnosis and management of refractorygastroesophagealrefluxdisease.” Gastroenterology&hepatology 17.7 (2021): 305. https://doi.org/10.1016/B978-0-323-99865-9.00001-4
7. Yasuda, Jessica L., et al. “Pharmacogenomicsfail to explainprotonpumpinhibitorrefractoryesophagitis in pediatricesophageal atresia.” Neurogastroenterology&Motility 34.1 (2022): e14217. doi:10.1111/nmo.14217
8. Lee, Christopher J., Timothy M. Farrell, and Evan S. Dellon. “TreatmentOutcomes of PatientsWithOverlappingEosinophilicEsophagitis and GastroesophagealRefluxDiseaseAfterAntirefluxSurgery.” Foregut (2024): 26345161241237521. doi:10.1177/26345161241237521
9. Krishnan, Usha. “Eosinophilicesophagitis in esophageal atresia.” Frontiers in pediatrics 7 (2019): 497. doi:10.3389/fped.2019.00497
10. Mabrouk, Mahasen Abd Alrahman, Shendy Mohammed Sherif, and Mahmoud Hassan Ali MorsyEliouny. “A StudyonEosinophilic and LymphocyticEsophagitis in PatientswithTypicalGastroesophagealRefluxDiseaseSymptoms.” International Journal of HealthSciences II: 3358-3371. doi:10.53730/ijhs.v6nS2.5858
11. Greuter, Thomas, et al. “Effectiveness and safety of high-vs low-doseswallowedtopicalsteroidsformaintenancetreatment of eosinophilicesophagitis: a multicenterobservationalstudy.” ClinicalGastroenterology and Hepatology 19.12 (2021): 2514-2523. doi:10.1016/j.cgh.2020.08.027
12. Hirsch, Suzanna, et al. “Characterization of eosinophilicesophagitis in infants and toddlers.” Journal of PediatricGastroenterology and Nutrition 77.1 (2023): 86-92. doi:10.1097/MPG.0000000000003803
13. Muftah, Mayssan, et al. “Baselineperipheraleosinophilcountindependentlypredictsprotonpumpinhibitor response in eosinophilicesophagitis.” Journal of ClinicalGastroenterology 58.3 (2024): 242-246. doi:10.1097/MCG.0000000000001845
14. Alaber, Omar, et al. “Epidemiology of eosinophilicesophagitis in patientswithcystic fibrosis: a population-based 5-year study.” PediatricGastroenterology, Hepatology&Nutrition 25.4 (2022): 283. doi:10.5223%2Fpghn.2022.25.4.283
15. Pesce, Marcella, et al. “Isthere a role for pH impedancemonitoring in identifyingeosinophilicesophagitis in childrenwithesophageal atresia?.” TheJournal of Pediatrics 210 (2019): 134-140. doi:10.1016/j.jpeds.2019.03.015
16. Muir, Amanda, and Gary W. Falk. “Eosinophilicesophagitis: a review.” Jama 326.13 (2021): 1310-1318. doi:10.1001/jama.2021.14920
17. Rawy, Abeer M., Amr M. Elmistekawy, and Rabie E. Elshaer. “Studyontheassociationbetweeneosinophilicesophagitis and bronchialasthma in Egyptianpatientswithesophagealsymptoms.” TheEgyptianJournal of ChestDiseases and Tuberculosis 69.2 (2020): 323-330. doi:10.4103/ejcdt.ejcdt_144_19
18. Ketchem, Corey J., et al. “Higherbodymassindexisassociatedwithdecreasedtreatment response to topicalsteroids in eosinophilicesophagitis.” ClinicalGastroenterology and Hepatology 21.9 (2023): 2252-2259. doi:10.1016/j.cgh.2022.11.004
19. Abulawi, Ahmad, et al. “High-ResolutionEsophagealManometricFeatures in EosinophilicEsophagitisPatients: A RetrospectiveStudy.” GastroHepAdvances 1.5 (2022): 703-708. doi:10.1016/j.gastha.2022.04.020
20. Menard-Katcher, Calies, and Seema Aceves. “Pathophysiology and ClinicalImpact of EsophagealRemodeling and Fibrosis in EosinophilicEsophagitis.” Immunology and AllergyClinics (2024).doi:10.1016/j.iac.2023.12.002
21. Visaggi, Pierfrancesco, et al. “Eosinophilicesophagitis: clinical, endoscopic, histologic and therapeuticdifferences and similaritiesbetweenchildren and adults.” Therapeuticadvances in gastroenterology 14 (2021): 1756284820980860. doi:10.1177/1756284820980860
22. Fujiwara, Yasuhiro. “Symptom-baseddiagnosticapproachforeosinophilicesophagitis.” Journal of gastroenterology 55 (2020): 833-845. doi:10.1007/s00535-020-01701-y
23. Biedermann, Luc, et al. “Eosinophilicesophagitis—establishedfacts and new horizons.” Seminars in Immunopathology. Vol. 43. No. 3. Berlin/Heidelberg: SpringerBerlin Heidelberg, 2021. doi:10.1007/s00281-021-00855-y
24. Grasso, Julianna, Diane RigassioRadler, and RenaZelig. “Single‐foodelimination of cow’smilk as a treatmentforeosinophilicesophagitis in childrenaged 2–18 years: A review of theliterature.” Nutrition in ClinicalPractice (2024). doi:10.1002/ncp.11117
25. Rothenberg, Marc E., et al. “Impressions and aspirationsfromthe FDA GREAT VI workshop oneosinophilic gastrointestinal disordersbeyondeosinophilicesophagitis and perspectivesforprogress in thefield.” Journal of Allergy and ClinicalImmunology 149.3 (2022): 844-853. doi:10.1016/j.jaci.2021.12.768.
FINANCING
None.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: Nithesh Babu Ramesh, Prasanna venkatesh, Tusha, Neha Nanditha Adepu, Abinaya Srinivasa Rangan, Shubhansh Bansal.
Data curation: Nithesh Babu Ramesh, Prasanna venkatesh, Tusha, Neha Nanditha Adepu, Abinaya Srinivasa Rangan, Shubhansh Bansal.
Formal analysis: Nithesh Babu Ramesh, Prasanna venkatesh, Tusha, Neha Nanditha Adepu, Abinaya Srinivasa Rangan, Shubhansh Bansal.
Research: Nithesh Babu Ramesh, Prasanna venkatesh, Tusha, Neha Nanditha Adepu, Abinaya Srinivasa Rangan, Shubhansh Bansal.
Drafting - original draft: Nithesh Babu Ramesh, Prasanna venkatesh, Tusha, Neha Nanditha Adepu, Abinaya Srinivasa Rangan, Shubhansh Bansal.
Writing - proofreading and editing: Nithesh Babu Ramesh, Prasanna venkatesh, Tusha, Neha Nanditha Adepu, Abinaya Srinivasa Rangan, Shubhansh Bansal.