doi: 10.56294/saludcyt20241241
SYSTEMATIC REVIEW AND META-ANALYSIS
Emerging Strategies in Thyroid Cancer Immunotherapy: A Comprehensive Systematic Review and Meta-Analysis of Clinical Outcomes
Estrategias emergentes en inmunoterapia del cáncer de tiroides: Una revisión sistemática exhaustiva y un metaanálisis de los resultados clínicos
Daniel Alejandro Estrella Cornejo1
![]() *, Meylin Yalitza
Carriel Alvarado2
*, Meylin Yalitza
Carriel Alvarado2 ![]() *, Norma Susana
Chávez Villagómez3
*, Norma Susana
Chávez Villagómez3 ![]() *, Alberto Dario
Díaz Parra3
*, Alberto Dario
Díaz Parra3 ![]() *, María Fernanda
Navas Espinosa4
*, María Fernanda
Navas Espinosa4 ![]() *
*
1Universidad de las Américas/ IESS hospital San Francisco, Endocrinology. Quito, Ecuador.
2Ministerio de Salud Pública, Medical Department. Vinces, Ecuador.
3Universidad Nacional de Chimborazo, Clinical Laboratory - Faculty of Health Sciences. Riobamba, Ecuador.
4Independent Investigator. Quito, Ecuador.
Cite as: Estrella Cornejo DA, Carriel Alvarado MY, Chávez Villagómez NS, Díaz Parra AD, Navas Espinosa MF. Emerging Strategies in Thyroid Cancer Immunotherapy: A Comprehensive Systematic Review and Meta-Analysis of Clinical Outcomes. Salud, Ciencia y Tecnología. 2024; 4:1241. https://doi.org/10.56294/saludcyt20241241
Submitted: 10-02-2024 Revised: 24-05-2024 Accepted: 24-07-2024 Published: 25-07-2024
Editor: Dr. William
Castillo-González
![]()
ABSTRACT
Introduction: the most prevalent endocrine cancer is thyroid cancer (TC), which has a low death rate despite a rising frequency. In order to assess the clinical results of novel immunotherapeutic approaches in TC, this systematic review and meta-analysis will concentrate on treatment-related adverse events (AEs), overall response rate (ORR), progression-free survival (PFS), and overall survival (OS).
Methods: a thorough search was done on PubMed, Embase, and ClinicalTrials.gov, covering research published between January 2018 and December 2023. The inclusion criteria were satisfied by 14 research, including a range of TC subtypes and study methodologies.
Results: the effectiveness of immunotherapy varied throughout TC subtypes. In advanced TC with PD-L1 positivity, pembrolizumab showed a 9 % ORR and a 7-month PFS. In advanced/metastatic TC, camrelizumab + famitinib demonstrated ORRs of 33,3 %-62,5 % and 8,4-month PFS. Patients who tested positive for PD-L1 had greater responses to spartalizumab (19 % ORR) in ATC. Combination treatments, such as pembrolizumab and lenvatinib, demonstrated encouraging outcomes in ATC and poorly differentiated thyroid cancer (PDTC), with 34,3 % ORRs and a significant increase in PFS. With the fixed-effects model, the pooled ORR was 40,8 % (95 % CI, 37,2 %-44,5 %), and with the random-effects model, it was 33,4 % (95 % CI, 20,8 %-48,9 %). Considerable heterogeneity (I2 = 94,4 %, p < 0,01) demonstrated varying treatment outcomes across several immunotherapy protocols.
Conclusion: immunotherapy has promise in the treatment of advanced tuberculosis, especially aggressive forms such as ATC, especially when used in combination regimens. Subsequent investigations have to concentrate on refining combination tactics and finding biomarkers for patient selection.
Keywords: Thyroid Cancer; Immunotherapy; Immune Checkpoint Inhibitors; PD-1 Inhibitors.
RESUMEN
Introducción: el cáncer endocrino más prevalente es el cáncer de tiroides (CT), que tiene una baja tasa de mortalidad a pesar de una frecuencia creciente. Con el fin de evaluar los resultados clínicos de los nuevos enfoques inmunoterapéuticos en el CT, esta revisión sistemática y meta-análisis se concentrará en los eventos adversos relacionados con el tratamiento (EA), la tasa de respuesta global (TRO), la supervivencia libre de progresión (SLP) y la supervivencia global (SG).
Métodos: se realizó una búsqueda exhaustiva en PubMed, Embase y ClinicalTrials.gov, que abarcó las investigaciones publicadas entre enero de 2018 y diciembre de 2023. Los criterios de inclusión fueron satisfechos por 14 investigaciones, incluyendo una gama de subtipos de CT y metodologías de estudio.
Resultados: la eficacia de la inmunoterapia varió a lo largo de los subtipos de CT. En CT avanzado con positividad de PD-L1, pembrolizumab mostró una ORR del 9 % y una SLP de 7 meses. En el CT avanzado/metastásico, camrelizumab + famitinib demostraron ORR del 33,3 %-62,5 % y una SLP de 8,4 meses. Los pacientes que dieron positivo para PD-L1 tuvieron mayores respuestas a espartalizumab (ORR del 19 %) en ATC. Los tratamientos combinados, como pembrolizumab y lenvatinib, demostraron resultados alentadores en el ATC y el cáncer de tiroides pobremente diferenciado (PDTC), con ORR del 34,3 % y un aumento significativo de la SLP. Con el modelo de efectos fijos, la ORR agrupada fue del 40,8 % (IC del 95 %, 37,2 %-44,5 %), y con el modelo de efectos aleatorios, del 33,4 % (IC del 95 %, 20,8 %-48,9 %). La considerable heterogeneidad (I2 = 94,4 %, p < 0,01) demostró resultados de tratamiento variables entre varios protocolos de inmunoterapia.
Conclusiones: la inmunoterapia es prometedora en el tratamiento de la tuberculosis avanzada, especialmente en las formas agresivas como la ATC, sobre todo cuando se utiliza en regímenes combinados. Las investigaciones posteriores deben centrarse en perfeccionar las tácticas de combinación y encontrar biomarcadores para la selección de pacientes.
Palabras clave: Cáncer de Tiroides; Inmunoterapia; Inhibidores de Puntos de Control Inmunitarios; Inhibidores de PD-1.
INTRODUCTION
Thyroid cancer represents around 1 % of all neoplasms and is the most common endocrine system cancer. Developmental abnormalities, follicular cell-derived neoplasms, thyroid C-cell-derived carcinoma, mixed medullary and follicular cell-derived carcinomas, salivary gland-type thyroid carcinomas, thyroid tumors of uncertain histogenesis, thymic tumors within the thyroid, and embryonal thyroid neoplasms are the eight distinct groups into which thyroid cancer is classified according to the World Health Organization’s 2022 classification of thyroid tumors.(1) In addition, there are seven distinct subtypes of follicular cell-derived neoplasms, each with its own special traits. The subtypes of thyroid cancer that fall under this category are as follows: anaplastic follicular cell-derived thyroid carcinoma (ATC), poorly differentiated thyroid carcinoma (PDTC), differentiated high-grade thyroid carcinoma (DHGTC), oncocytic carcinoma of the thyroid (OCA), follicular thyroid carcinoma (FTC), and invasive encapsulated follicular variant papillary thyroid carcinoma (IEFVPTC). (1) The illness mostly affects younger people, that is, those between the ages of 16 and 33, and it is more common in women.(2) Prolonged exposure to ionizing radiation, obesity, Hashimoto’s thyroiditis, high iodine consumption, and a family history of thyroid cancer are important risk factors.(3)
With an anticipated 44,000 new cases in 2022, TC is the 12th most frequent cancer diagnosis overall and the 7th most prevalent for women in the US.(4) The incidence of TC in women is about three times higher than in males. Furthermore, the incidence rate rises with age; for women, the average age at diagnosis is 52, and for males, it is 69. (4)Despite a notable rise in the prevalence of this malignancy throughout the last three decades, the death rate has been quite low, hovering at 0,5 per 100,000 cases. ATCs are more aggressive, with a historical overall survival of just four to six months, and account for 40–50 % of all TC-related fatalities in the United States. In contrast, DTCs have a positive prognosis with a 98,4 % 5-year relative survival.(5)
Even with these developments, it is still critical to look for new, efficient therapies for high-grade thyroid tumors. With a variety of techniques including adoptive immunotherapy, monoclonal antibodies, cancer vaccines, tumor-associated macrophage targeting, and immune checkpoint blocking, immunotherapy has emerged as a potentially effective treatment, especially for anaplastic thyroid carcinoma.(6) Notably, clinical studies have shown the therapeutic effectiveness of anti-PD-1 antibody spartalizumab in treating locally advanced or metastatic ATC, and the FDA approved pembrolizumab for the treatment of thyroid cancer in 2020.(7)
Wang et al.’s bibliometric evaluation emphasizes the post-2018 research boom and the field’s quick progress and increasing interest in thyroid cancer immunotherapy.(8) For advanced MTC and ATC, immunocheckpoint inhibitors are advised, especially in late phases of therapy. Despite great progress, no systematic review has evaluated the clinical performance of these new immunotherapies for thyroid cancer in detail. In order to close this gap, a comprehensive review and meta-analysis of modern immunotherapeutic methods’ safety and effectiveness is conducted. The main outcomes of interest are overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and treatment-related adverse events (AEs). The goal of the study is to provide thorough insights that will direct future investigations and practical applications in the treatment of thyroid cancer.
METHODS
Study Design:
This systematic review and meta-analysis were designed to evaluate emerging strategies in thyroid cancer immunotherapy and their clinical outcomes.
Sample Selection:
The search strategy aimed to systematically identify relevant studies on thyroid cancer immunotherapy. A combination of keywords and MeSH terms with Boolean operators (AND, OR) was used in databases such as PubMed, Embase, and ClinicalTrials.gov. The search terms included “thyroid cancer” OR “thyroid carcinoma” AND “immunotherapy” OR “checkpoint inhibitors” OR “PD-1 inhibitors” AND “clinical trial” OR “cohort study” OR “treatment outcomes”.
Inclusion criteria:
Studies investigating the efficacy and safety of immunotherapy in thyroid cancer.
Research articles written in English.
Studies involving human subjects.
Publications from January 2018 to December 2023.
Articles published in peer-reviewed journals.
Studies providing clear methodologies and data on clinical outcomes such as ORR, PFS, OS, and safety.
Exclusion criteria:
Review articles, case reports and series, editorials, letters, and conference abstracts.
Studies focusing solely on preclinical or laboratory findings without clinical outcomes.
Studies not specifically examining immunotherapy in thyroid cancer.
Non-English articles.
Study Method:
An extensive electronic search for articles was conducted, and studies were evaluated based on their abstracts and titles. The full texts of selected articles were then thoroughly assessed, adhering to specific inclusion and exclusion criteria. Only articles meeting the inclusion criteria were included in the review.
Data Extraction:
Data were extracted from selected studies using a standardized form. Key information including study characteristics (e.g., author, publication year), study design, population type and number, intervention details, outcomes assessed, and results with statistics were systematically recorded.
Data Analysis:
To synthesize the quantitative information from the included research, a meta-analysis was done. The pooled ORR was the main result, and it was determined using both fixed-effect and random-effects models. With values more than 50 % indicating significant heterogeneity, the I2 statistic and Cochran’s Q test were used to evaluate the heterogeneity among the studies. The DerSimonian-Laird estimator was used in the meta-analysis to estimate the between-study variance (tau2) using the inverse variance approach with a logit transformation for proportions. For each study’s proportions, Clopper-Pearson confidence intervals were used. Subgroup and sensitivity analyses were performed to evaluate the robustness of the results and investigate the causes of heterogeneity.
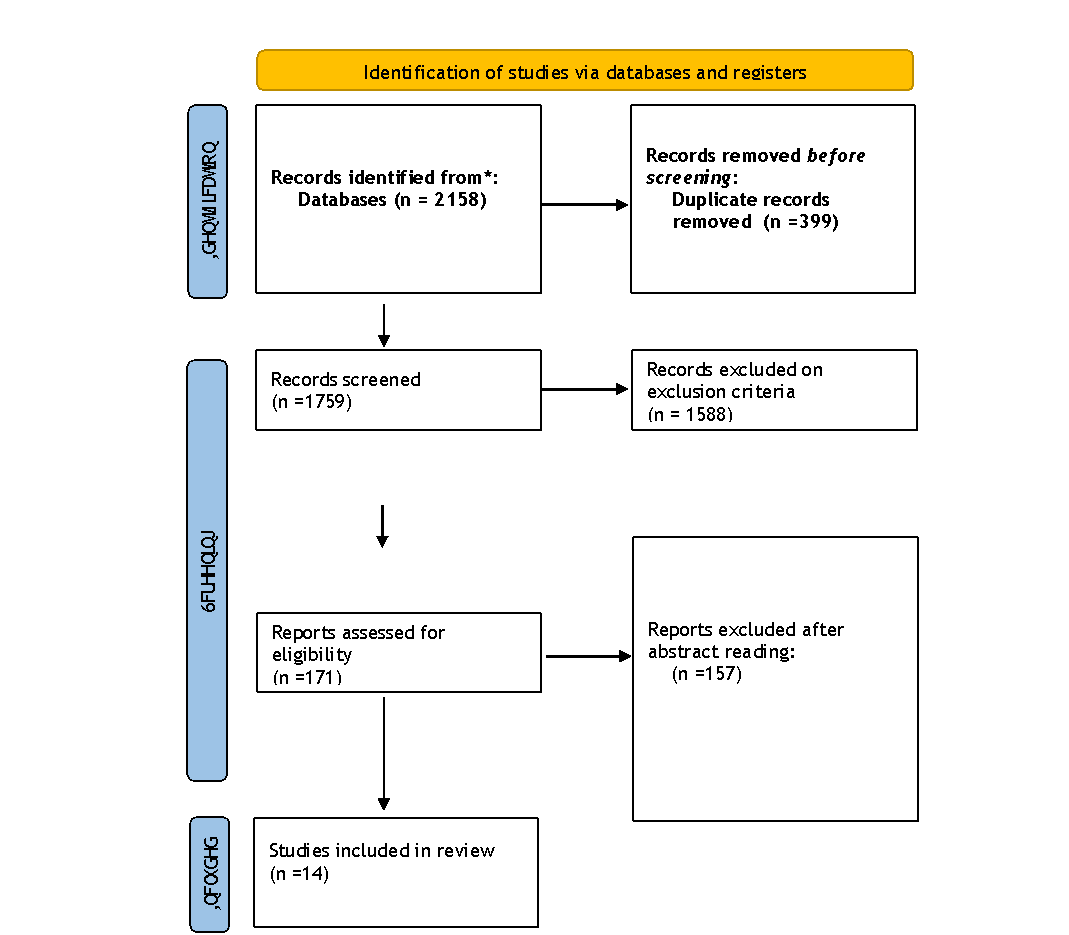
Figure 1. Prima Flow Diagram
RESULTS
In the first round of the literature search, 2158 publications were found. After abstracts and titles were carefully evaluated, 171 publications were found to be relevant, and their complete texts were obtained for further analysis. Studies that were excluded either did not specifically look at immunotherapy for thyroid cancer or did not meet the inclusion criteria. Fourteen publications were determined to be suitable for the systematic review and meta-analysis after an extensive screening process.
Study characteristics:
The comprehensive review of novel approaches in thyroid cancer immunotherapy included 14 clinical investigations. Phase II single-arm trials (n = 5, 36 %), phase II clinical trials (6 = 2, 43 %), phase Ib nonrandomized investigations (n = 1, 7 %), and retrospective cohort studies (n = 2, 14 %) are among the many different designs covered by these studies.
Thyroid cancer subtypes included in the studies included medullary thyroid cancer (MTC), radioiodine-refractory differentiated thyroid cancer (RAIR DTC), advanced/metastatic anaplastic thyroid carcinoma (ATC), and recurrent/metastatic cervical squamous cell carcinoma. The patient populations in the studies were diverse. There was a large variation in sample sizes, with values ranging from 5 to 103 cases.
The majority of the interventions included the combination of new medicines including spartalizumab, camrelizumab, and toripalimab, as well as checkpoint inhibitors like pembrolizumab, nivolumab, and ipilimumab. These agents were often used in conjunction with kinase inhibitors or other chemotherapeutic treatments. Objective response rate (ORR), progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and safety profiles were among the frequently evaluated outcomes.
|
Table 1. Characteristics and the results of the studies reviewed |
|||||||
|
Author Name |
Year |
Study Type |
Population Type and Number |
Intervention |
Outcomes Assessed |
Results with Stats |
Conclusion |
|
Janice M. Mehnert et al,(9) |
2019 |
Phase Ib, Nonrandomized clinical trial |
Advanced, PD-L1–positive papillary or follicular thyroid cancer; n=22 |
Pembrolizumab 10 mg/kg every 2 weeks up to 24 months |
ORR, PFS, OS, safety |
ORR: 9 % (95 % CI, 1-29 %); PFS: 7 months (95 % CI, 2-14 months); OS: not reached; Treatment-related AEs in 82 % of patients |
Pembrolizumab has a manageable safety profile and demonstrates evidence of antitumor activity in a minority of patients with advanced differentiated thyroid cancer. |
|
Dongmei Ji et al,(10) |
2022 |
single-arm, open-label phase II study |
Advanced/metastatic thyroid cancer; n=74 |
Camrelizumab 200 mg i.v. + Famitinib 20 mg p.o. daily |
ORR, DCR, PFS, OS, safety |
ORR: 33,3 %-62,5 % across different groups; DCR: 96 %-100 %; PFS: 8,4 months (Group 4); OS: 13,6 months (Group 4); AEs: diarrhea (37 %), fatigue (31,5 %) |
Camrelizumab plus famitinib shows promising antitumor efficacy with acceptable toxicity in patients with advanced or metastatic thyroid cancer. |
|
Lingfang Xia et al, (11) |
2022 |
Phase II, clinical trial |
Recurrent/metastatic cervical squamous cell carcinoma; n=33 |
Camrelizumab 200 mg i.v. + Famitinib 20 mg p.o. daily |
ORR, DOR, DCR, TTR, PFS, OS, safety |
ORR: 39,4 % (95 % CI, 22,9-57,9); DOR: 74,1 % at 12 months; PFS: 10,3 months (95 % CI, 3,5-not reached); OS: 77,7 % at 12 months; AEs: 100 %, serious AEs in 27,3 % |
Camrelizumab plus famitinib shows promising antitumor activity with a manageable and tolerable safety profile in pretreated recurrent/metastatic cervical squamous cell carcinoma. |
|
Jaume Capdevila et al, (12) |
2020 |
Phase II clinical trial |
Advanced/metastatic anaplastic thyroid carcinoma; n=42 |
Spartalizumab 400 mg i.v. every 4 weeks |
ORR, safety |
ORR: 19 %; CR: 3 patients; PR: 5 patients; Higher response in PD-L1-positive (29 %) vs PD-L1-negative (0 %); 1-year survival: 52,1 % in PD-L1-positive patients |
Spartalizumab shows responsiveness in anaplastic thyroid carcinoma, especially in PD-L1-positive patients, with durable responses and a manageable safety profile. |
|
C. Dierks et al, (13) |
2022 |
Phase II clinical trial |
Metastasized anaplastic and poorly differentiated thyroid carcinoma; n=35 |
Lenvatinib 20 mg daily + Pembrolizumab 200 mg i.v. every 3 weeks |
ORR, PFS, OS, safety |
ORR at 3 months: 34,3 %; ATC: PR 51,9 %, SD 44,4 %; PDTC: PR 75 %, SD 25 %; Median PFS: ATC 9,5 months, PDTC 20 months; OS: ATC 10,25 months, PDTC not reached |
Lenvatinib and pembrolizumab combination is effective and safe, inducing high response rates and long-lasting remissions in patients with ATC and PDTC. |
|
Priyanka C. Iyer et al, (14) |
2018 |
Retrospective cohort study |
ATC patients progressed on kinase inhibitors; n=12 |
Pembrolizumab + kinase inhibitors at progression |
BOR, PFS, OS, safety |
BOR: PR 42 %, SD 33 %, PD 25 %; Median OS from start of KI: 10,43 months; OS from addition of pembrolizumab: 6,93 months; PFS from addition of pembrolizumab: 2,96 months |
Pembrolizumab combined with kinase inhibitors may be an effective salvage therapy in ATC patients who progress on kinase inhibitors, warranting further exploration of frontline combination strategies. |
|
Nancy Y. Lee et al, (15) |
2022 |
single-arm clinical trial, |
Metastatic anaplastic thyroid cancer; n=12 |
Tremelimumab 75 mg q4 weeks + Durvalumab 1500 mg q4 weeks + SBRT 9 Gy × 3 fractions |
1-year OS, treatment duration, median OS |
Median OS: 14,5 weeks; 1-year OS: 1 patient; 0 confirmed responses; 1 patient had stable disease; Median treatment duration: 11 weeks |
Tremelimumab and durvalumab with SBRT did not improve OS for metastatic ATC. Future research needed for other immunotherapy combinations with/without radiotherapy. |
|
Bryan Haugen et al, (16) |
2020 |
Single-arm Phase II, clinical trial |
RAIR differentiated thyroid cancer; n=30 |
Lenvatinib 20 mg/day + Pembrolizumab 200 mg every 3 weeks |
CR, ORR, PFS, safety |
ORR: 62 % PR, 35 % SD; Median PFS: not reached; 12-month PFS: 74 %; Median treatment duration: 9,9 months; 70 % required lenvatinib dose reduction |
Lenvatinib plus pembrolizumab is reasonably tolerated and has a high ORR in RAIR DTC patients. No documented complete responses. |
|
Sophie Leboulleux et al, (17) |
2021 |
Phase II, Open-label clinical trial |
Radioactive iodine refractory thyroid cancer; n=43 (DTC: 27, ATC: 16) |
Pembrolizumab 200 mg every 3 weeks |
OR, duration of response, PFS, OS, safety |
DTC: PR 11,1 %, SD 18,5 %, median PFS: 2,6 months, median OS: 12,7 months; ATC: PR 18,8 %, SD 6,2 %, median PFS: 2,3 months, median OS: 3,6 months |
Pembrolizumab shows low response rates in DTC and moderate in ATC with short duration of response. Notable but short-lived efficacy in ATC. |
|
Do-Youn Oh et al, (18) |
2023 |
Phase II, Multi-cohort clinical trial |
Advanced thyroid cancer; n=103 |
Pembrolizumab 200 mg every 3 weeks |
ORR, duration of response, PFS, OS, safety |
ORR: 6,8 % (95 % CI, 2,8 %-13,5 %); Median duration of response: 18,4 months; Median PFS: 4,2 months (95 % CI, 3,9-6,2); Median OS: 34,5 months (95 % CI, 21,2 to not reached); Treatment-related AEs: 69,9 % (Grade 3-5: 14,6 %) |
Pembrolizumab shows modest antitumor activity and manageable toxicity in advanced thyroid cancer regardless of PD-L1 expression. Future studies should focus on biomarker-driven patient selection or combination therapies. |
|
Jochen H. Lorch et al. (19) |
2020 |
Phase II clinical trial |
RAIR DTC (n=32), ATC (n=10), MTC (n=7); Total n=49 |
Nivolumab 3mg/kg every 2 weeks + Ipilimumab 1mg/kg every 6 weeks |
Radiographic response rate (CR+PR), PFS, TRAEs |
DTC: 9,4 % PR (95 % CI, 2 %-25 %); ATC: 30 % PR (95 % CI, 7 %-65 %); MTC: 0 % PR; TRAEs Grade 3-4: increased lipase (n=8), increased serum amylase (n=4), treatment-related adrenal insufficiency (n=4) |
Nivolumab and ipilimumab have considerable activity in ATC with notable PRs. Activity in unselected RAIR DTC is low but responses were seen in PDTC and Hurthle cell TC. Exceptional responses with prolonged remissions were observed. |
|
Jiaying Chen et al. (20) |
2022 |
Single-arm Phase II, clinical trial |
Locally advanced thyroid cancer; n=10 |
Surufatinib 250 mg daily + Toripalimab 240 mg every 3 weeks |
ORR, R0/1 resection rate, DCR, time to remission (TTR), AEs |
ORR: 66,7 % (4 PR, 2 SD); 4 PR and 1 SD patients received R0 resections; No Grade ≥3 AEs observed; Safety profile consistent with previously reported data |
Surufatinib combined with toripalimab is feasible and well-tolerated as neoadjuvant therapy for locally advanced thyroid cancer, achieving majority R0 resections. Further investigation is warranted based on good preliminary results. |
|
Jia-Ying Chen et al. (21) |
2023 |
Single-arm Phase II, clinical trial |
Locally advanced DTC; n=10 |
Surufatinib 250 mg daily + Toripalimab 240 mg every 3 weeks |
ORR, R0/1 resection rate, AEs |
ORR: 60 %; 9 patients received R0/1 resections; Median best percentage change in target lesion diameter: 32 %; Most AEs were Grade 1 or 2 |
Surufatinib combined with toripalimab as neoadjuvant therapy is feasible, achieving majority R0/1 resection in locally advanced DTC. It represents a new treatment option needing further investigation. |
|
Dominik Soll et al. (22) |
2024 |
Retrospective cohort study |
Advanced anaplastic thyroid cancer (ATC); n=5 |
Lenvatinib (14-24 mg daily) + Pembrolizumab 200 mg every 3 weeks |
PFS, OS, response rate, AEs |
Median PFS: 4,7 months (range 0,8-5,9); Median OS: 6,3 months (range 0,8-not reached); 1 PR, 3 SD, 1 not evaluable due to acute infectious thyroiditis; PD-L1 CPS: 12-100 % |
Lenvatinib and pembrolizumab showed effectiveness and moderate tolerability in treatment-naïve ATC patients with occasional long-lasting responses. However, exceptional responses seen in pretreated patients were not confirmed. |
Meta analysis:
The objective response rate (ORR) of novel immunotherapeutic approaches for thyroid cancer was assessed in nine trials by means of a meta-analysis. Treatments including pembrolizumab, camrelizumab, spartalizumab, lenvatinib, and toripalimab were evaluated in the pooled analysis. An ORR of 40,8 % (95 % CI, 37,2 %-44,5 %) was calculated by the fixed-effect model, while an ORR of 33,4 % (95 % CI, 20,8 %-48,9 %) was obtained using the random-effects model. Significant heterogeneity was shown in the trials (I2 = 94,4 %, p < 0,01), showing that the various immunotherapy regimens had diverse therapeutic responses. Interestingly, ORRs varied from 7 % to 67 %, indicating different levels of efficacy in different clinical contexts and combinations of treatments. This significant variability highlights the need for further study and individualized methods to improve immunotherapy techniques for thyroid cancer.
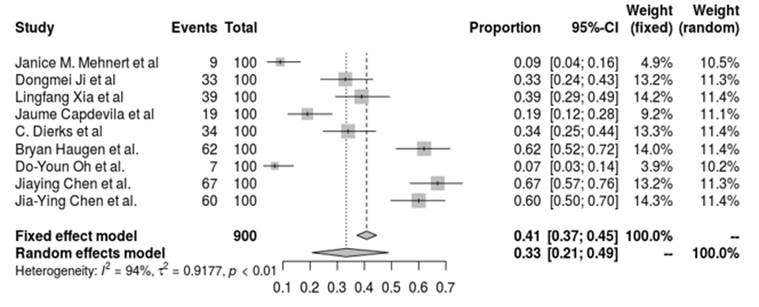
Source: Own elaboration
DISCUSSION
The extensive evaluation and
meta-analysis of fourteen research provide important new perspectives on the
therapeutic results and developing strategies for immunotherapy against thyroid
cancer. This corpus of work includes a range of immunotherapeutic medications,
including as toripalimab, lenvatinib, spartalizumab, pembrolizumab, and
camrelizumab, as well as their combinations with other treatments such kinase
inhibitors and radiation therapy.
The differences in objective response rates (ORRs) across these trials show the
heterogeneity of thyroid cancer and its response to immunotherapy. Mehnert et
al, for example, found that pembrolizumab had a 9 % ORR in patients with
advanced thyroid cancer who were positive for PD-L1, suggesting that the
treatment had limited efficacy in this group.(9) Ji et al., on the
other hand, reported ORRs ranging from 33,3 % to 62,5 % when camrelizumab and
famitinib were taken together, indicating a more potent antitumor impact. The
advantages of combination therapies in enhancing patients’ therapeutic results
for thyroid cancer are shown by this comparison.(10)
In advanced/metastatic anaplastic thyroid cancer, Capdevila et al. reported an ORR of 19 % with spartalizumab; patients who were PD-L1-positive (29 %) showed higher efficacy than those who were PD-L1-negative (0 %).(12) The significance of PD-L1 expression as a gauge of immunotherapy response is highlighted by this discovery. In a similar vein, research by Dierks et al. and Haugen et al. showed ORRs of 34,3 % and 62 %, respectively, for lenvatinib and pembrolizumab combined, indicating that these combinations take use of synergistic effects to provide better clinical results than monotherapies.(13)
A fixed-effects model yielded an ORR of 40,8 % and a random-effects model of 33,4 %, respectively, in the pooled analysis, demonstrating significant heterogeneity in treatment responses across various clinical situations and medication combinations. Considerable heterogeneity (I2 = 94,4 %, p < 0,01) was noted, which is indicative of the variety of clinical circumstances and therapies that were assessed.
The immunotherapies had generally acceptable safety profiles, but the number of adverse events (AEs) varied. According to Haugen et al., adverse events (AEs) involving pembrolizumab and lenvatinib combinations resulted in dose reductions for 70 % of patients.(16) Common AEs included tiredness, diarrhea, and raised lipase and amylase levels. Ji et al. similarly observed that of individuals taking camrelizumab plus famitinib, 37 % had diarrhea and 31,5 % had weariness. These results emphasize the need of meticulous AE monitoring and management in order to maximize treatment compliance and results.(10)
Notably, Lee et al. discovered that in patients with metastatic anaplastic thyroid carcinoma, the combination of tremelimumab, durvalumab, and SBRT produced no validated responses and a median overall survival (OS) of just 14,5 weeks, highlighting the need for alternate strategies.(15) Numerous studies have reaffirmed the importance of PD-L1 expression in predicting the responsiveness to immunotherapy. Capdevila et al., for instance, reported improved outcomes in PD-L1-positive individuals receiving spartalizumab treatment.(12) On the other hand, pembrolizumab demonstrated a modest level of anticancer activity independent of PD-L1 expression, as reported by Oh et al, indicating the need for additional biomarkers to improve patient selection.(18)
By using synergistic effects, combination treatments have shown better clinical results in thyroid cancer immunotherapy as compared to monotherapies. With camrelizumab plus famitinib, Ji et al. obtained outstanding ORRs (33,3 % to 62,5 %) and a disease control rate (DCR) of 96 %-100 %.(10) The most responsive group saw an OS of 13,6 months and a median progression-free survival (PFS) of 8,4 months. In poorly differentiated thyroid cancer (PDTC) and metastasized anaplastic thyroid carcinoma (ATC), Dierks et al. reported an ORR of 34,3 % with lenvatinib and pembrolizumab, with a median progression-free survival (PFS) of 20 months for PDTC and 9,5 months for ATC.(13) Lenvatinib and pembrolizumab were used to treat radioactively iodine-refractory differentiated thyroid carcinoma (RAIR DTC) with a 62 % ORR and a 74 % 12-month PFS rate, according to Haugen et al. However, 70 % of patients needed dose decreases because of side effects.(16)
In ATC patients treated with pembrolizumab with kinase inhibitors who had progressed on prior therapies, Iyer et al. observed a best overall response (BOR) of 42 % partial response (PR), suggesting its potential as an effective salvage therapy.(14) With a median OS of just 14,5 weeks, Lee et al. observed the poor effectiveness of SBRT in combination with durvalumab and tremelimumab for metastatic ATC. Low response rates to pembrolizumab in thyroid cancer that is radioactively iodine-refractory was noted by Leboulleux et al., indicating the need for combination regimens to increase effectiveness.(17) On the other hand, Chen et al. showed that surufatinib and toripalimab have potential as neoadjuvant treatments for thyroid cancer that has progressed locally.(20) They were able to achieve an ORR of 66,7 %, with successful resections of R0 and no adverse events of Grade 3 or above.
There are a few drawbacks to combination treatments for thyroid cancer immunotherapy, despite their encouraging outcomes. The intricacy of research methodologies, patient demographics, and treatment plans poses challenges to the direct comparability and broad applicability of the results. Small sample sizes and single-arm designs were common in this research, which might have introduced biases and reduced the results’ robustness. Furthermore, adverse effects linked to combination therapies—like those seen in trials combining pembrolizumab and lenvatinib—call for cautious treatment and emphasize the need of toxicity mitigation techniques. Large-scale randomized controlled trials should be the main focus of future research in order to verify these results and investigate biomarkers for more effective patient selection. Optimizing dosage schedules and looking at new combinations might improve therapeutic effectiveness and safety even further. Furthermore, customizing medicines to each patient’s unique profile via the use of genetic and molecular profiling in clinical practice may enhance results and reduce side effects.
CONCLUSION
In conclusion, this systematic
review and meta-analysis demonstrate the encouraging prospects of immunotherapy
for the treatment of advanced thyroid cancer, especially in cases of aggressive
types like anaplastic thyroid carcinoma (ATC). The results suggest that various
thyroid cancer subtypes have varying degrees of effectiveness, with combination
regimens exhibiting significantly greater rates of response and longer
progression-free survival (PFS) in comparison to monotherapies. Immunotherapies
such as pembrolizumab, camrelizumab, and spartalizumab show considerable
clinical advantages and tolerable safety profiles, despite the notable
variation in treatment results. This is particularly true when paired with
kinase inhibitors or chemotherapeutics. In order to improve treatment
effectiveness and outcomes in the therapy of thyroid cancer, our data highlight
the need for more research to optimize combination methods and find predictive
biomarkers for improved patient selection.
REFERENCES
1. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach. Endocrinol Metab. 2022;37(5):703. doi:10.3803/ENM.2022.1553
2. Haymart MR. Progress and Challenges in Thyroid Cancer Management. Endocr Pract. 2021;27(12):1260-1263. doi:10.1016/J.EPRAC.2021.09.006
3. Myung SK, Lee CW, Lee J, Kim J, Kim HS. Risk Factors for Thyroid Cancer: A Hospital-Based Case-Control Study in Korean Adults. Cancer Res Treat. 2017;49(1):70. doi:10.4143/CRT.2015.310
4. Turner N, Hamidi S, Ouni R, et al. Emerging therapeutic options for follicular-derived thyroid cancer in the era of immunotherapy. Front Immunol. 2024;15. doi:10.3389/FIMMU.2024.1369780
5. Maniakas A, Dadu R, Busaidy NL, et al. Evaluation of Overall Survival in Patients with Anaplastic Thyroid Carcinoma, 2000-2019. JAMA Oncol. 2020;6(9):1397-1404. doi:10.1001/JAMAONCOL.2020.3362
6. Ma M, Lin B, Wang M, et al. Immunotherapy in anaplastic thyroid cancer. Am J Transl Res. 2020;12(3):974. Accessed June 25, 2024. /pmc/articles/PMC7137046/
7. Haddad RI, Bischoff L, Ball D, et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2022;20(8):925-951. doi:10.6004/JNCCN.2022.0040
8. Wang K, Zhang Y, Xing Y, Wang H, He M, Guo R. Current and future of immunotherapy for thyroid cancer based on bibliometrics and clinical trials. Discov Oncol. 2024;15(1). doi:10.1007/S12672-024-00904-6
9. Mehnert JM, Varga A, Brose MS, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer. 2019;19(1). doi:10.1186/S12885-019-5380-3
10. Ji D, Shen W, Kuang M, et al. A phase II study to evaluate the efficacy and safety of camrelizumab plus famitinib in advanced or metastatic thyroid cancer. https://doi.org/101200/JCO20224016_suppl6085. 2022;40(16_suppl):6085-6085. doi:10.1200/JCO.2022.40.16_SUPPL.6085
11. Xia L, Zhou Q, Gao Y, et al. A multicenter phase 2 trial of camrelizumab plus famitinib for women with recurrent or metastatic cervical squamous cell carcinoma. Nat Commun. 2022;13(1). doi:10.1038/S41467-022-35133-4
12. Capdevila J, Wirth LJ, Ernst T, et al. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol. 2020;38(23):2620-2627. doi:10.1200/JCO.19.02727/SUPPL_FILE/PROTOCOL_JCO.19.02727.PDF
13. Dierks C, Ruf J, Seufert J, et al. 1646MO Phase II ATLEP trial: Final results for lenvatinib/pembrolizumab in metastasized anaplastic and poorly differentiated thyroid carcinoma. Ann Oncol. 2022;33:S1295. doi:10.1016/J.ANNONC.2022.07.1726
14. Iyer PC, Dadu R, Gule-Monroe M, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunother Cancer. 2018;6(1). doi:10.1186/S40425-018-0378-Y
15. Lee NY, Riaz N, Wu V, et al. A Pilot Study of Durvalumab (MEDI4736) with Tremelimumab in Combination with Image-Guided Stereotactic Body Radiotherapy in the Treatment of Metastatic Anaplastic Thyroid Cancer. Thyroid. 2022;32(7):799-806. doi:10.1089/THY.2022.0050
16. Haugen B, French J, Worden FP, et al. Lenvatinib plus pembrolizumab combination therapy in patients with radioiodine-refractory (RAIR), progressive differentiated thyroid cancer (DTC): Results of a multicenter phase II international thyroid oncology group trial. 2020;38(15_suppl):6512-6512. doi:10.1200/JCO.2020.38.15_SUPPL.6512
17. Leboulleux S, Godbert Y, Penel N, et al. Benefits of pembrolizumab in progressive radioactive iodine refractory thyroid cancer: Results of the AcSé Pembrolizumab Study from Unicancer. https://doi.org/101200/JCO20213915_suppl6082. 2021;39(15_suppl):6082-6082. doi:10.1200/JCO.2021.39.15_SUPPL.6082
18. Oh DY, Algazi A, Capdevila J, et al. Efficacy and safety of pembrolizumab monotherapy in patients with advanced thyroid cancer in the phase 2 KEYNOTE-158 study. Cancer. 2023;129(8):1195-1204. doi:10.1002/CNCR.34657
19. Lorch JH, Barletta JA, Nehs M, et al. A phase II study of nivolumab (N) plus ipilimumab (I) in radioidine refractory differentiated thyroid cancer (RAIR DTC) with exploratory cohorts in anaplastic (ATC) and medullary thyroid cancer (MTC). 2020;38(15_suppl):6513-6513. doi:10.1200/JCO.2020.38.15_SUPPL.6513
20. Chen J, Ji Q, Wang Y, et al. The efficacy and safety of anti–PD-1 antibody toripalimab combined with surufatinib in neoadjuvant treatment of locally advanced thyroid cancer: A phase II study. 2022;40(16_suppl):6084-6084. doi:10.1200/JCO.2022.40.16_SUPPL.6084
21. Chen J ying, Huang N si, Wei W jun, et al. The Efficacy and Safety of Surufatinib Combined with Anti PD-1 Antibody Toripalimab in Neoadjuvant Treatment of Locally Advanced Differentiated Thyroid Cancer: A Phase II Study. Ann Surg Oncol. 2023;30(12):7172-7180. doi:10.1245/S10434-023-14031-Z
22. Soll D, Bischoff P, Frisch A, et al. First effectiveness data of lenvatinib and pembrolizumab as first-line therapy in advanced anaplastic thyroid cancer: a retrospective cohort study. BMC Endocr Disord. 2024;24(1):1-9. doi:10.1186/S12902-024-01555-Y/TABLES/2
FINANCING
No financing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Daniel Alejandro Estrella Cornejo, Meylin Yalitza Carriel Alvarado.
Data curation: Norma Susana Chávez Villagómez, Alberto Dario Díaz Parra.
Formal analysis: María Fernanda Navas Espinosa, Daniel Alejandro Estrella Cornejo.
Methodology: Daniel Alejandro Estrella Cornejo, Meylin Yalitza Carriel Alvarado, Norma Susana Chávez Villagómez.
Project management: Alberto Dario Díaz Parra, María Fernanda Navas Espinosa.
Supervision: Alberto Dario Díaz Parra, María Fernanda Navas Espinosa, Daniel Alejandro Estrella Cornejo.
Validation: Meylin Yalitza Carriel Alvarado, Norma Susana Chávez Villagómez, Daniel Alejandro Estrella Cornejo.
Display: Norma Susana Chávez Villagómez, Alberto Dario Díaz Parra.
Drafting - original draft: María Fernanda Navas Espinosa, Daniel Alejandro Estrella Cornejo, Meylin Yalitza Carriel Alvarado.
Writing - proofreading and editing: Meylin Yalitza Carriel Alvarado, Norma Susana Chávez Villagómez, María Fernanda Navas Espinosa.