doi: 10.56294/saludcyt20241328
ORIGINAL
Mortality of cases with chronic kidney disease and its risk factors admitted to hospital with COVID-19 in Almaty city, Kazakhstan
Mortalidad de los casos con enfermedad renal crónica y sus factores de riesgo ingresados en el hospital con COVID-19 en la ciudad de Almaty, Kazajstán
Garazhayeva Laura1 ![]() *, Gaipov Abduzhappar2
*, Gaipov Abduzhappar2 ![]() *, Kauysheva Almagul3
*, Kauysheva Almagul3 ![]() *
*
1Kazakhstan Medical University «KSPH», Department of Medicine. Almaty, Kazakhstan.
2School of Medicine at the Nazarbayev University. Astana, Kazakhstan.
3Kazakhstan Medical University «KSPH», Department of Public Health. Almaty, Republic of Kazakhstan.
Cite as: Garazhayeva L, Abduzhappar G, Almagul K. Mortality of cases with chronic kidney disease and its risk factors admitted to hospital with COVID-19 in Almaty city, Kazakhstan. Salud, Ciencia y Tecnología. 2024; 4:1328. https://doi.org/10.56294/saludcyt20241328
Submitted: 18-02-2024 Revised: 10-05-2024 Accepted: 20-07-2024 Published: 21-07-2024
Editor:
Dr.
William Castillo-González ![]()
ABSTRACT
Objetive: to determine the rate of mortality of cases with chronic kidney disease and its risk factors admitted to hospital with COVID-19 in Almaty City, Kazakhstan.
Methods: patients with coronavirus infections who were hospitalized at a hospital in the Kazakhstani Almaty region from June 2020 until June 2022 are included in the retrospective analysis. The Unified National Electronic Healthcare System (UNEHS) provided the database extraction. Individuals were considered eligible if they had been admitted to the hospital with the primary diagnoses of U07.1 (COVID-19, detected virus) and U07.2.
Conclusion: in Almaty, Kazakhstan, hospitalization outcomes for coronavirus patients with and without chronic kidney disease were assessed in this study. Analysis was done on the impact of comorbidities and sociodemographic characteristics on mortality. While there are many ways to prevent and manage chronic kidney disease (CKD), coexisting medical diseases, particularly viruses that cause pandemics, might complicate matters. Thus, to prevent unplanned infectious disease outbreaks, a thorough disease management plan must be established.
Keywords: Chronic Kidney Disease; Mortality; Immunity; Haemodialysis; SARS–Cov–2.
RESUMEN
Objetivo: demostrar la tasa de mortalidad de los casos con enfermedad renal crónica y sus factores de riesgo ingresados en el hospital con COVID-19 en la ciudad de Almaty, Kazajstán.
Método: se incluyen en el análisis retrospectivo los pacientes con infecciones por coronavirus que fueron hospitalizados en un hospital de la región kazaja de Almaty desde junio de 2020 hasta junio de 2022. El Sistema Nacional Unificado de Atención Médica Electrónica (UNEHS) proporcionó la extracción de la base de datos. Los individuos se consideraron elegibles si habían sido ingresados en el hospital con los diagnósticos primarios de U07.1 (COVID-19, virus detectado) y U07.2.
Conclusiones: en Almaty, Kazajistán, se evaluaron en este estudio los resultados de la hospitalización de pacientes con coronavirus con y sin enfermedad renal crónica. Se analizó el impacto de las comorbilidades y las características sociodemográficas en la mortalidad. Aunque hay muchas formas de prevenir y tratar la enfermedad renal crónica (ERC), las enfermedades médicas coexistentes, en particular los virus que causan pandemias, pueden complicar las cosas. Por lo tanto, para prevenir brotes imprevistos de enfermedades infecciosas, debe establecerse un plan exhaustivo de gestión de la enfermedad.
Palabras clave: Enfermedad Renal Crónica; Mortalidad; Inmunidad; Hemodiálisis; SARS-Cov-2.
INTRODUCTION
Age is a major predictor of CKD, with approximately 38 % of patients being over 65. The adaptive and innate immune systems' typical response is compromised in patients with chronic kidney disease (CKD). As a result, viral infections and chronic concomitant illnesses are more common in this patient cohort. The COVID-19 pandemic changed CKD patients' morbidity and mortality rates. According to the study, those with renal impairment were more likely than those without CKD to experience worse results from a coronavirus infection. This study looks into the in-hospital mortality of coronavirus-positive CKD patients in Almaty, Kazakhstan.(1)
The prevalence of chronic kidney disease, which frequently goes undiagnosed by cases and medical personnel, ranges between 8 % and 16 % worldwide.(1,2,3) The most common causes of chronic kidney disease mentioned globally are hypertension and/or diabetes, but many developing countries in Asia and sub-Saharan Africa also have high rates of infection, glomerulonephritis, and environmental exposures (like air pesticides, herbal medicines, and pollution). Genetic risk factors may also have an impact on chronic kidney disease risk. People with African heritage, but not those with European ancestry, are more likely to have the sickle cell trait and to carry the two APOL1 risk alleles.(4)
The majority of the time, chronic kidney disease is only identified by chance or by regular testing with urine and serum chemistry profiles. Less common symptoms that individuals may have include gross hematuria, "foamy urine" (an indicator of albuminuria), flank pain, nocturia, and decreased urine production.(5) Dyspnea, peripheral edoema, low appetite, exhaustion, vomiting, nausea, metallic taste, unintentional weight loss, pruritus, and changes in mental status are other symptoms that cases with severe chronic kidney disease may encounter.(6)
The highly contagious condition known as coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has had a devastating effect on the world, killing almost 6 million people. When the first instances of this primarily respiratory viral infection were identified in Wuhan, Hubei Province, China, towards the end of December 2019, SARS-CoV-2 rapidly spread throughout the world. On March 11, 2020, the World Health Organisation (WHO) had no choice but designate it a global pandemic due to this.(7)
A cytokine storm caused by infection by a virus, which results in a prothrombotic state and systemic inflammation, is thought to be the pathogenesis of COVID-19, which is characterised by an excess of inflammatory cytokines.(7) This raises questions concerning the clinical outcomes and prognosis of cases who are receiving immunosuppressive medication and have preexisting comorbidities such as end-stage kidney disease, CKD, and kidney transplant recipients.(8) Patients with SARS-CoV-2 infection also experience difficulties with other organs, like kidney dysfunction that results in acute kidney injury (AKI), in addition to lung involvement.(9,10)
Research aim
The objective of our research is to determine the death rate and risk variables associated with chronic renal disease in patients admitted to hospitals in Almaty City, Kazakhstan, who have COVID-19.
Literature review
Epidemiology
Coronaviruses (CoVs) are positive-sense, single-stranded RNA viruses that are enfolded and range in size from 60 to 140 nm, or 100 times smaller than a typical human cell. Under the electron microscope, the surface outgrowths' spike-like appearance gave rise to the term coronavirus. These viruses can infect mammals, including humans and birds, and cause diseases of the respiratory, nervous, hepatic, and gastrointestinal systems. There are six CoVs known to be pathogenic.(11) The four genera of the CoVs are α-, β-, γ-, and δ-CoVs. While the α- and β-CoVs can infect mammals, they typically impact birds.(12) These deadly CoVs periodically appear in various locations.(13) The same functional receptor as SARS-CoV, angiotensin-converting enzyme 2 (ACE2), appears to be the route through which SARS-CoV-2 penetrates host cells.(14)
Pathogenesis
The SARS-CoV-2 strain of the CoV was discovered in Wuhan cases who had unexplained pneumonia and were isolated from their lower respiratory tracts. Healthcare facilities and community areas have shown SARS-CoV-2 transfer from one person to another, especially among individuals in close quarters.(15) Infection can happen when a person touches a contaminated surface and introduces virus-carrying droplets to their lips, eyes, or nose. SARS-CoV and MERS-CoV can both infect the human gastrointestinal tract, and SARS-CoV has been observed to have the potential to spread via faeces to humans.(15,16,17)
The upper airways' epithelial cells, including those lining the bronchi and nasal passages, are the ones that SARS-CoV-2 primarily targets for infection.(18) By slicing activating the S protein and ACE2, the type II transmembrane serine protease (TMPRSS2), which is present on the host cell in a superficial manner, promotes viral uptake. SARS-CoV-2 entrance into host cells is mediated by activation of the S protein (figure 1).(18,19)
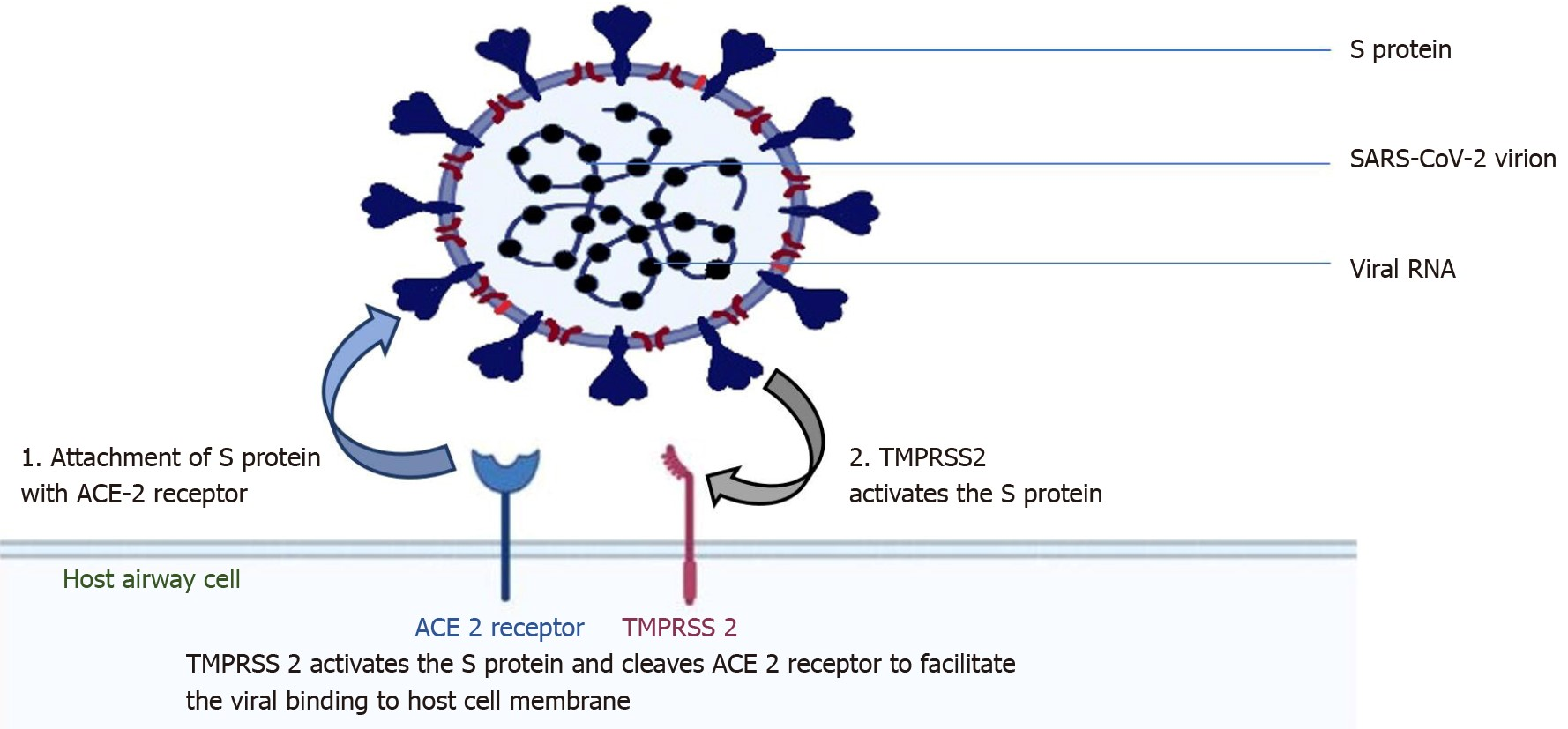
Figure 1. Acute respiratory distress of severe magnitude
Angiotensin- converting enzyme 2 (ACE2) receptor-binding viral structure S protein is the target of coronavirus 2; 2: type 2 transmembrane serine protease, a host cell, facilitates viral entry by activating the S protein and cleaving ACE2. ACE2 is angiotensin-converting enzyme 2; SARS-CoV-2 is the severe acute respiratory syndrome coronavirus 2; and TMPRSS2 is type 2 transmembrane serine protease.(22)
Depending on the variant that caused the symptoms, the median times for admission to the intensive care unit (ICU), mechanical ventilation, acute respiratory distress syndrome (ARDS), dyspnea, and admission at the hospital differed slightly. However, an examination of the order in which the events occurred indicates that the original virus's median times were 7,0, 10,5, 8,0, 9,0, and 10,5 days, respectively. This lends more credence to the theory that severe CO is caused by an immunological response that is dysregulated (figure 2, 3).(21)
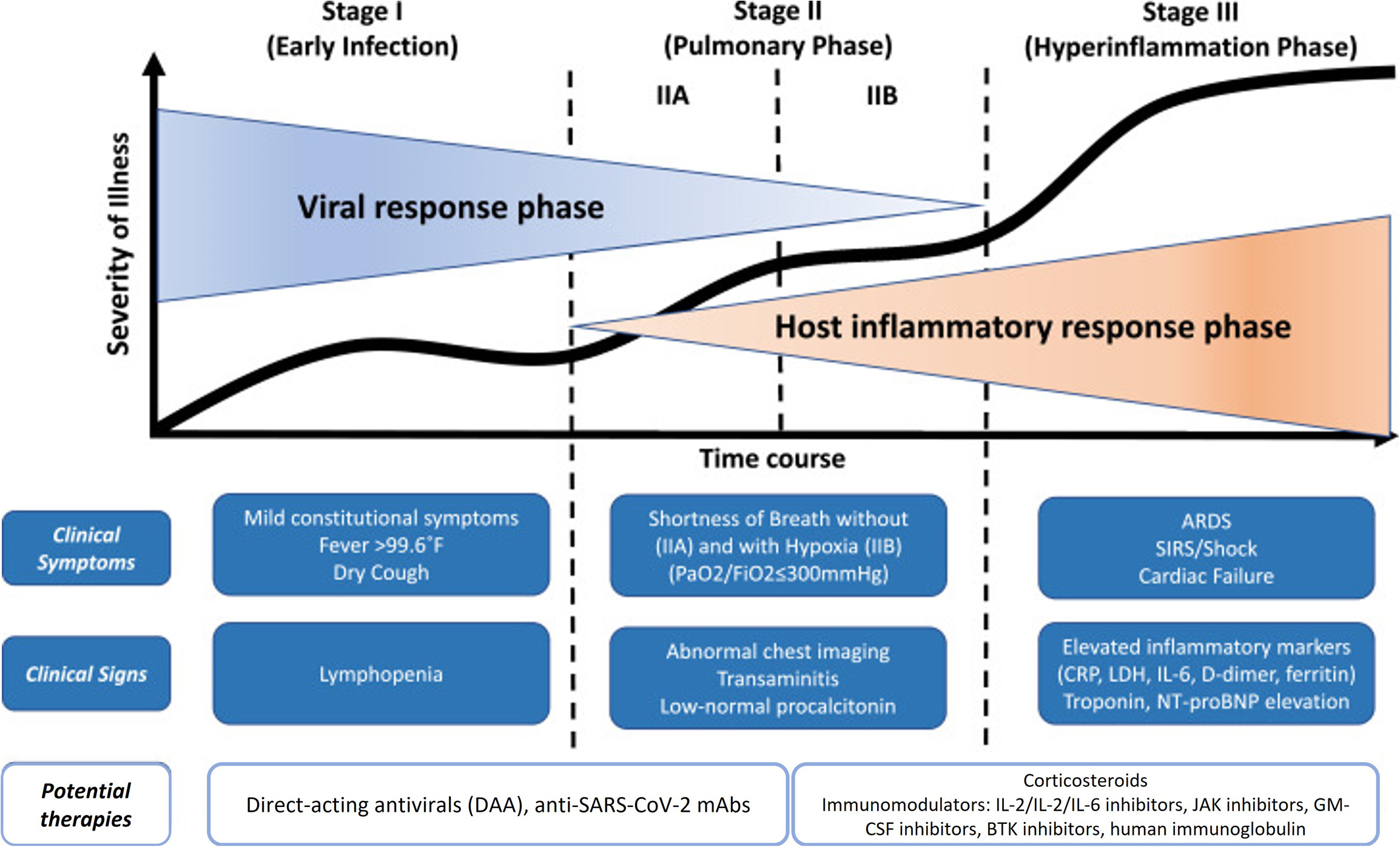
Figure 2. Stages of SARS-CoV-2 infection, how they relate to clinical symptoms and found therapeutic treatments(23)
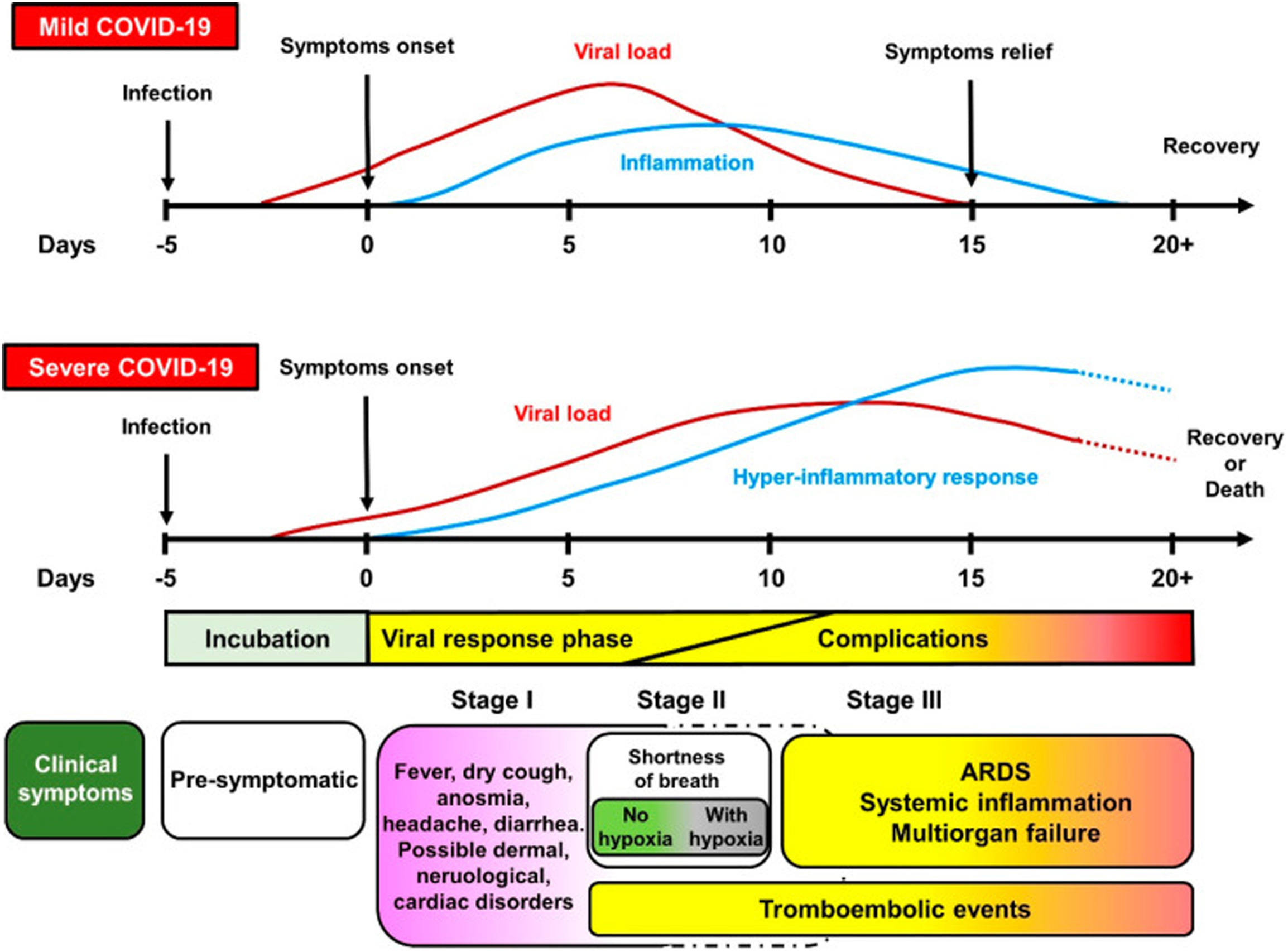
Figure 3. Timeline of COVID-19, both mild and severe, and its relationship to the activity of the virus and symptoms(23)
Innate Immune Response
When host receptors detect pathogen-associated molecular patterns (PAMPs), the innate immune response is triggered. Small molecules known as PAMPs, including lipopolysaccharides, peptidoglycans, nucleic acids, and lipoteichoic acid, are present in a variety of patterns and, when recognized by the host, set off immunological cascades.(24) Pattern recognition receptors are the name given to the protein receptors in the host that recognize PAMPs.(25) Once engaged, these receptors initiate signaling cascades that stimulate other inflammasome complexes, such as the NOD-like receptor P3 inflammasome, and secrete proinflammatory cytokines, such as IL-18 and IL-1, in addition to releasing type III and type I interferons. The inflammasome pathway initiates the coagulation cascade and is also involved in the thrombotic events and coagulopathy observed in severe COVID-19 cases.(26)
In cases of necrosis, inflammation, or hypoxia, wounded or stressed host cells can still activate host cells even in the absence of microbial PAMPs. These are molecular patterns that are related to damage. Damage-associated molecular pattern mechanisms and activated PAMPaid in virus eradication, but an overreaction to immunotherapy can upset the immune system's equilibrium and exacerbate damage and inflammation by inducing a cytokine storm. For viral infections, these processes have previously been documented; however, SARS-CoV-2-specific comparable pathways have not yet been found.(27)
The increased frequency of reports of ARDS in individuals suffering from severe COVID-19 raised awareness of one proinflammatory cytokine, IL-6. Adaptive and innate immune responses are mediated by IL-6, a myokine that is both pro- and anti-inflammatory. Macrophages release IL-6 in response to PAMPs binding to receptors of the pattern recognition. A lot of attention was put on treating severe COVID-19 with antagonists of the IL-6 receptor because it was shown that an increased IL-6 was linked to a bad prognosis. In severe COVID-19, clinical trials have indicated that inhibiting IL-6 may help to moderate overreactive immune responses.(28) from getting started and increases the likelihood of COVID-19 becoming severe or fatal (figure 4).(29)
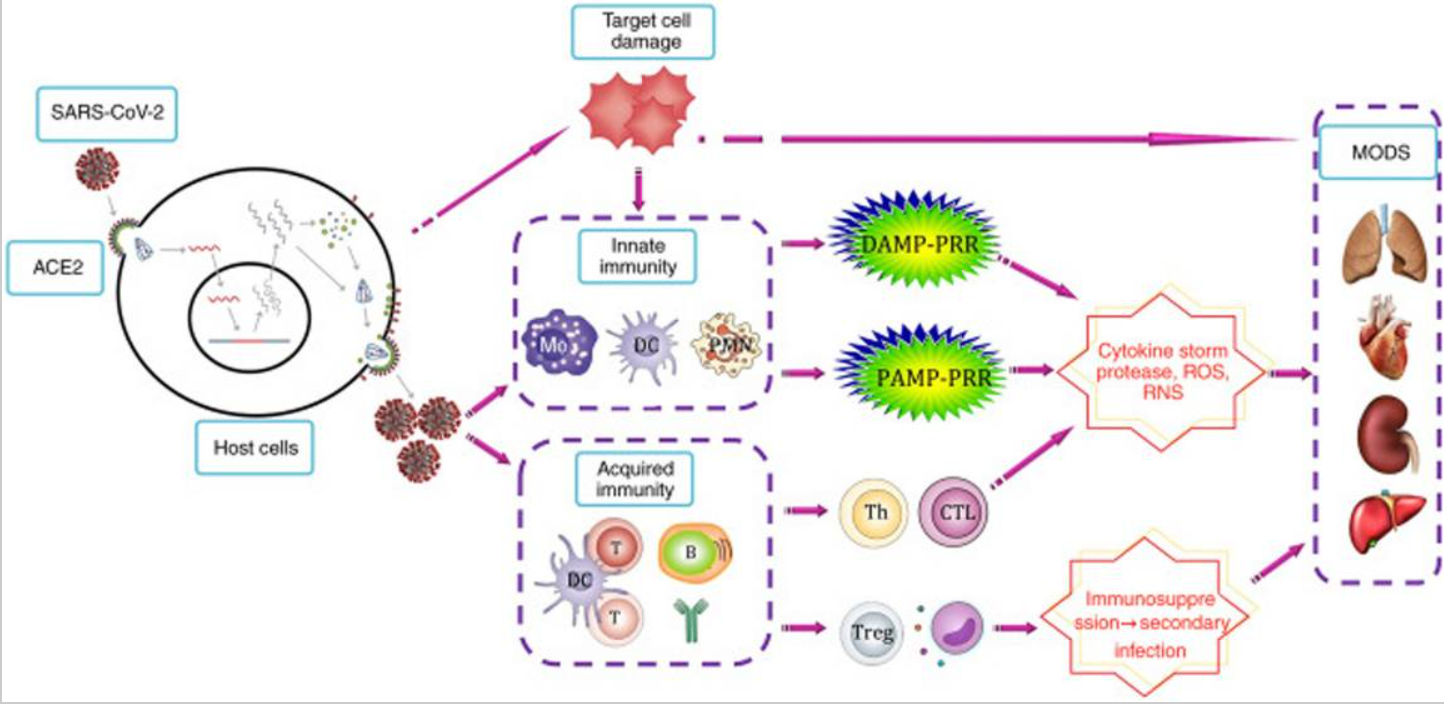
Figure 4. The aetiology of infection by SARS-CoV-2 and the response of the immunity to it(23)
An early innate immune response is critical to trigger the immune systems of T- and B-cells and terminate the infection at the mild-to-moderate stage or asymptomatic stage. Defective host immunity or immunological evasion by the virus (or both) can cause an insufficient or missing response of the innate immunity response, which prevents the adaptive immune system.
Diagnostic Testing in COVID-19
The usual diagnostic test is a nasopharyngeal swab for SARS-CoV-2 nucleic acid utilizing a real-time PCR technique. The FDA in the USA has approved commercial PCR assays for the qualitative identification of the SARS-CoV-2 virus using samples taken from nasopharyngeal swabs as well as oropharyngeal, nasopharyngeal aspirates, anterior/mid-turbinate nasal swabs, bronchoalveolar lavage (BAL), and saliva.(30)
The variables that affect the sensitivity of PCR testing involve the specimen's adequacy, the duration following exposure, and the material's source. Nonetheless, the bulk of commercially available SARS-CoV-2 PCR tests with FDA authorization have a specificity of about 100 % provided there is no chance of cross-contamination during specimen preparation. SARS-CoV-2 antigen assays are less sensitive than molecular PCR testing, although they can be completed more quickly.(31)
Despite the large number of antibody tests that have been produced to date, serologic testing has limitations with respect to sensitivity and specificity. Additionally, the results of different tests may vary. The NIH guidelines advise against using serologic testing to identify the presence of acute SARS-CoV-2 infection. Additionally, they claimed that the evidence was insufficient to support either a recommendation for or against the use of serologic testing to evaluate immunity, even if it was utilized in guiding clinical choices about COVID-19 vaccines and monoclonal antibodies.(32)
Clinical characteristics
Signs of SARS-CoV-2 infection may not be obvious. The most prevalent clinical signs are fatigue/tiredness (38,1 %), pyrexia (88,7 %), cough (67,8 %), dyspnea (18,6 %), sputum production (33,4 %), headache (13,6 %), and sore throat (13,9 %). It was discovered that some people, in particular, had asymptomatic illnesses or were afebrile. The respiratory, gastrointestinal, neurologic, gastrointestinal (diarrhea, nausea, and vomiting), respiratory (cough, rhinorrhea, chest discomfort, sore throat, shortness of breath, and hemoptysis), musculoskeletal (myalgia), and gastrointestinal systems are among those that are affected.(33,34)
Complications
The majority of adult COVID-19 cases have a favorable outlook; however, those who are older than 60 or who have underlying chronic illnesses, such as lung disease, obesity, diabetes, or hypertensive heart disease, are more likely to feel a severe or critical illness as a result of COVID-19. Poor clinical outcomes and disease severity are strongly associated, and disease progression is more likely to be accelerated in older people. Additionally, the interval between the start of symptoms and mortality is shorter among cases who are older (> 65 years old). Immune systems may be compromised in newborns and the elderly, demanding special care (figure 5).(35,36)
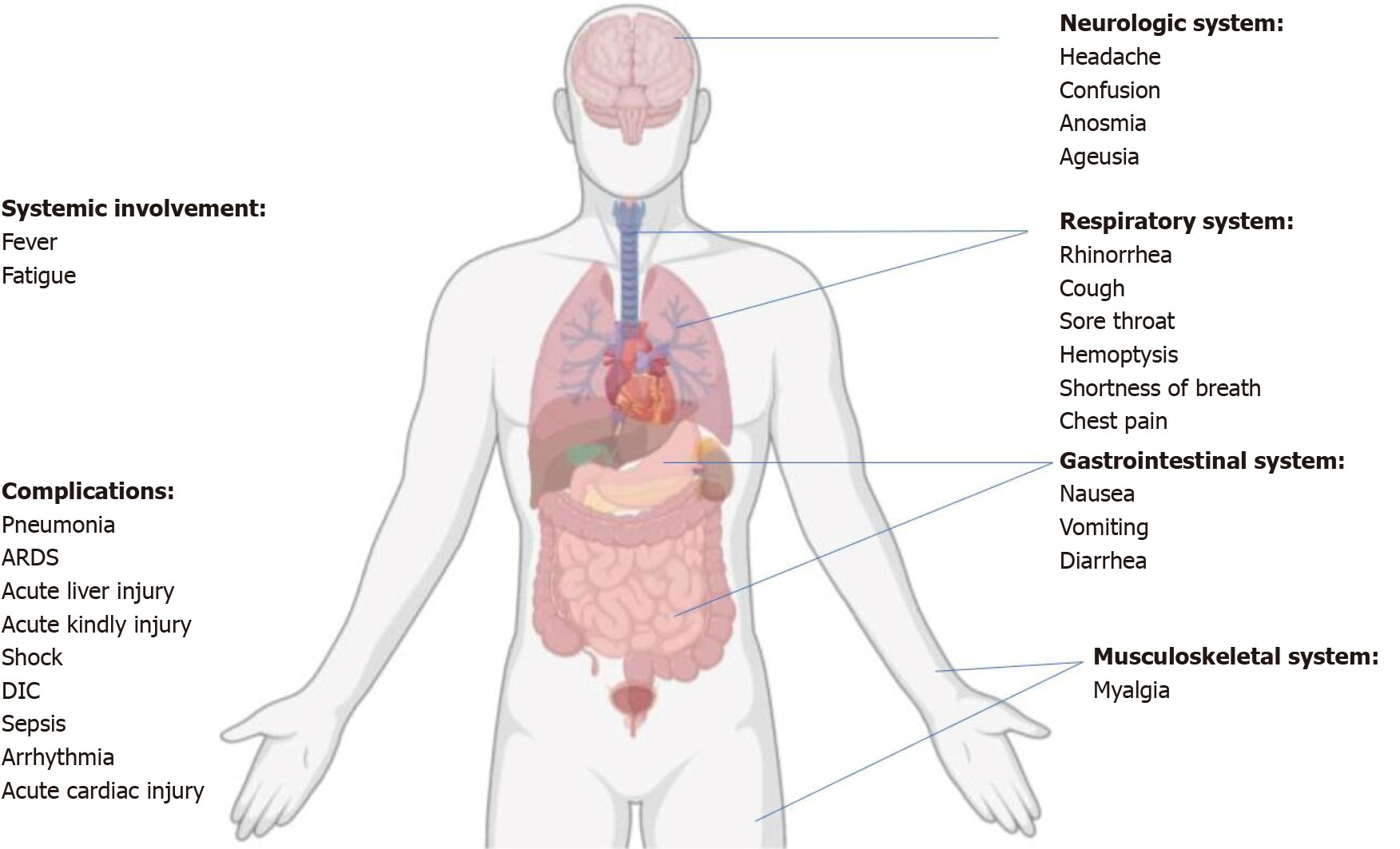
Figure 5. Summary of the coronavirus disease's symptoms, systemic manifestations, and complications in 2019. Disseminated intravascular coagulation (DIC) is a condition similar to acute respiratory distress syndrome (ARDS)(22)
Post-COVID-19 problems affect 10 to 20 percent of COVID-19 virus victims. 80-85 % of cases with post-COVID-19 symptoms report continued symptoms after a year of follow-up, according to longitudinal studies.(37,38,39) Post-COVID syndrome's natural history and range of consequences are not well understood because of how recent it is. In contrast to cases with CKD who did not need renal replacement therapy or those who had undergone kidney or pancreas/kidney transplantation, those receiving maintenance dialysis showed a greater incidence of COVID-19. Patients with CKD were more at risk than those with type 2 diabetes or congestive heart failure.(40,41)
Manifestations of chronic kidney disease
If kidney damage happens gradually, signs and markers of chronic renal illness emerge progressively over time. Electrolyte imbalances, body waste accumulation, and fluid retention can all be consequences of kidney failure.(42) Kidney disease can produce anorexia, weakness, nausea, vomiting, and tiredness, depending on how severe it is. Sleep problems, approximately urinating, diminished mental clarity, bodily cramping, ankle and foot swelling. The signs and symptoms of hypertension include dry, itchy skin, shortness of breath, trouble controlling high blood pressure (hypertension), and chest pain if fluid builds up around the walls of the heart. The signs and symptoms of kidney illness are usually ambiguous.
Both the innate and the adaptive immune systems are impacted by chronic kidney disease. The innate system, which is propelled by dendritic antigen-presenting cells (APC), macrophages, and polymorphs, is a quick, reliable, and ubiquitous method of defense against pathogens. Activated T and B cells power the adaptive immune system that is antigen-specific and necessitates the identification of processed antigens. Table 1 displays a summary of immune system abnormalities.(43) Patients with chronic kidney disease are more likely to contract infections, develop malignancies linked to viruses, or have a reduced response to vaccinations if the innate and adaptive immune systems are compromised. On the other hand, this group of individuals may potentially have an aberrant, heightened immune response that causes increased synthesis and impaired clearance of proinflammatory cytokines, which can result in inflammation and its aftereffects (such as atherosclerotic cardiovascular disease). Because of this, cases with COVID-19 infection and CKD appear to be at higher risk of hospitalization than those without chronic kidney disease (table 1).(44)
|
Table 1. An overview of how uremia affects the innate and adaptive immune systems(44) |
|
|
Disturbances in ESRD |
|
|
Innate immunity Pattern recognition receptors Endocytic Secreted Cells Neutrophils Monocytes
Complement |
Down-regulated Decreased
bactericidal abilities Ineffective
production to prevent infections Increased Th1/Th2
ratio |
COVID-19 and chronic kidney disease
Both hospitalized and outpatient COVID-19 cases have an increased risk of developing AKI due to chronic kidney disease, and both conditions can advance to renal failure. The presence of an undetected chronic kidney disease before hospitalization may have contributed to the COVID-19 infection. The prevalence of chronic kidney disease is 11 % worldwide, and it is significantly greater in low-income continents like Africa, where it ranges from 13,9 % to 15,8 %.(45)
Those who survived COVID-19 may experience long-term kidney problems. 6 months after being hospitalized for COVID-19, a Chinese study found that the estimated glomerular filtration rate had decreased by 35 %. Even though there was no indication of AKI at the time of admission, 13 % of cases had decreased estimated glomerular filtration rates. After AKI, many cases' creatinine levels return to normal, but the kidneys may not fully heal, raising the risk of chronic kidney disease over time.(46) In COVID-19, the progression of kidney disease is probably complex and may be triggered by ongoing inflammation, intrinsic tubular lesions, or inadequate repair. Due to the lack of KRT and higher costs associated with unfavorable outcomes, kidney failure necessitating dialysis increases mortality in the majority of cases with chronic kidney disease in LICs. Aging, diabetes mellitus, hypertension, a lack of a sufficient nephrology staff, and a lack of proper diagnostic tools are risk factors linked to a worse result with chronic kidney disease.(47)
In low-income settings, there hasn't been much success achieved in raising awareness of the need to manage and avoid kidney disease risk factors. In addition, non-communicable diseases like diabetes and hypertension that can cause kidney disease are becoming more common and are responsible for 80 % of fatalities in LLMICs globally.(48) To reduce the burden of noncommunicable illnesses and conditions that affect kidney health, governments must make more investments in health. A national plan for chronic kidney disease prevention should include public health initiatives like education, a good diet, and exercise.(49) Government support has been inadequate in this area, with few long-lasting initiatives to lessen the burden of kidney illness. Individuals and some nonprofit organizations, such as the ISN's World Kidney Day activities, have pushed for such public health initiatives. With or without COVID-19, such programs should be supported to decrease the burden of renal illness. Based on the decline in SARS-CoV-2 transmission, the burden of renal illness among COVID-19 cases has decreased. In order to avoid AKI and chronic kidney disease, it is imperative that vaccines be optimized globally, with early case detection and suitable care.(50)
The Republic of Kazakhstan's data on the incidence and clinical trajectory of COVID-19 have not yet been thoroughly gathered and studied. There have been some reports regarding COVID-19 infections in Kazakhstani children and the general population as of late.(51) Given that COVID-19 is a new disease, it is of considerable theoretical and practical importance to study all aspects of disease manifestation and outcomes in the population of Kazakhstan.
It should be mentioned that the most common symptoms among COVID-19 cases in the Kazakh population were runny nose, fever, sore throat, and cough. Recent studies have found that some syndromic symptoms, such as a dry cough, muscle soreness, exhaustion, shortness of breath, and anorexia, are more conclusive signs that a patient has COVID-19 than the presence of a sore throat, vomiting, a runny nose, diarrhea, nausea, and loss of consciousness.(52,53)
METHOD
General background
Approximately 9–12 % of people have CKD. Age is one of the key indicators of chronic kidney disease, and roughly 38 % of cases are beyond the age of 65. The innate and adaptive immune systems' typical response is impaired in people with CKD. As a result, this group of individuals is more susceptible to viral infections and persistent comorbid illnesses. According to numerous research, CKD is one of the major risk factors for severe COVID-19 and unfavorable outcomes. According to estimates, the prevalence of CKD in COVID-19 cases ranges from 0,09 to 47,05 %. Therefore, it is crucial to comprehend COVID-19 epidemiology in order to ascertain how it affects chronic kidney disease cases, manage such cases, develop customized health protection policies, and implement effective COVID-19 vaccine programs. AKI, which is a common complication of COVID-19 in addition to ARDS, is largely caused by tissue inflammation, localized immune cell infiltration, endothelial injuries, and micro-vascular clots. The much greater incidence of COVID-19 in individuals with CKD is most likely explained by the so-called pro-inflammatory cytokine storm syndrome and the oxidative stress that determines lung inflammation. In this scoping review, we investigated the COVID-19 effect on CKD.
We included individuals who, between June 2020 and June 2022, had been hospitalized in the Kazakhstani Almaty region due to coronavirus infection.
Data collection and Information sources
The Unified National Electronic Healthcare System (UNEHS) provided the database extraction. Those who had been admitted to the hospital with the primary diagnoses of U07.2 (COVID-19, virus not identified) and U07.1 (COVID-19, virus identified) were considered patients. The database of individuals with CKD was merged using the distinct IDs of the patients to determine whether or not they had the condition.
Statistical analysis
Patients who have been hospitalized in the Kazakhstani Almaty region from June 2020 until June 2022 due to coronavirus infection are included in the retrospective analysis. STATA 16.0 was used to conduct the statistical analysis. For bivariate analysis, Person's chi-square test was employed, and logistic regression was utilized to estimate the association between in-hospital mortality and predictors.
RESULTS
We used our search method to do a search that produced 6820 articles. The articles that were pertinent to our theme were then chosen after we had screened the rest. We performed a full-text screening of 288 publications after excluding those that did not pass the title and abstract screening. In the end, 63 publications were used to compile data for our topic and compose this review (figure 6).

Figure 6. Results of our search
929 people (2 %) out of the 58 970 patients in the final cohort had CKD. Table 2 indicates that there was a statistically significant age difference between the kidney disease patients and the comparator group. Comparing CKD patients to the reference group, they showed a greater ratio of comorbid diseases, including diabetes, hypertension, acute myocardial infarction (AMI), cerebrovascular disease (CVD), and congestive heart failure (CHF). In the two groups, there was a statistically significant difference in the death ratio.
|
Table 2. Patients' comorbidities and demographics, both with and without CKD |
||||
|
Characteristic |
Total (n = 58,970) |
Do not have CKD (n = 58,041, 98 %) |
Do have CKD (n = 929, 2 %) |
p-value |
|
Age, years old |
52 (±21) |
51 (±21) |
65 (±16) |
<0,001 |
|
Age category, n (%) |
|
|
|
|
|
- ≤34 y.o. |
13,294 (22) |
13,230 (23) |
64 (7) |
<0,001 |
|
- 35 - 50 y.o. |
11,763 (20) |
11,673 (20) |
90 (10) |
|
|
- 51 - 70 y.o. |
22,751 (39) |
22,373 (39) |
378 (41) |
|
|
- ≥71 y.o. |
11,162 (19) |
10,765 (18) |
397 (42) |
|
|
Gender, n (%) |
|
|
|
0,062 |
|
- Female |
34,957 (59) |
34,434 (59) |
523 (56) |
|
|
- Male |
24,013 (41) |
23,607 (41) |
406 (44) |
|
|
Acute myocardial infarction, n (%) |
|
|
|
<0,001 |
|
- No |
57,343 (97) |
56,535 (97) |
808 (87) |
|
|
- Yes |
1,627 (3) |
1,506 (3) |
121 (13) |
|
|
Diabetes, n (%) |
|
|
|
<0,001 |
|
- No |
53,779 (91) |
53,112 (92) |
667 (72) |
|
|
- Yes |
5,191 (9) |
4,929 (8) |
262 (28) |
|
|
Hypertension, n (%) |
|
|
|
<0,001 |
|
- No |
43,459 (74) |
43,251 (75) |
208 (22) |
|
|
- Yes |
15,511 (26) |
14,790 (25) |
721 (78) |
|
|
Congestive heart failure, n (%) |
|
|
|
<0,001 |
Unadjusted logistic regression showed that those with CKD, AMI, diabetes, hypertension, CHF, and CVD, as well as those who were elderly and male, had increased mortality risks (figure 7). When the aforementioned indicators were taken into account, CVD, age, CKD, male gender, and diabetes all showed statistically significant increases in mortality risks.

Figure 7. Relationship between a patient's in-hospital death and their sociodemographic and medical variables
DISCUSSION
Clinical outcomes in cases with chronic kidney disease with COVID-19 have been reported in single-center trials with wildly varying results. This diversity is partly attributable to patient factors like age, race, sex, and comorbidities that are linked to poorer outcomes. However, other variables, including as variations in the pressure placed on medical facilities by high numbers of COVID-19 cases, heterogeneity in data collection methods, techniques used to determine and categorise chronic kidney disease stage and clinical outcomes, and study time points, have an impact on reported results. In order to limit causes of variability, large multicenter trials with a wide variety of medical centres spread across vast geographic areas are required.
Post-COVID-19 problems affect 10 to 20 percent of COVID-19 virus victims. 80-85 % of cases with post-COVID-19 symptoms report continued symptoms after a year of follow-up, according to longitudinal studies. Post-COVID syndrome's natural history and range of consequences are not well understood because of how recent it is. In contrast to cases with CKD who did not need renal replacement therapy or those who had undergone kidney or pancreas/kidney transplantation, those receiving maintenance dialysis showed a greater incidence of COVID-19. Patients with chronic kidney disease were more at risk than those with type 2 diabetes or congestive heart failure.(37,38,39)
This is supported by our findings, which show that COVID-19-infected chronic kidney disease cases had a greater rate of mortality than COVID-19-uninfected cases (pooled OR 5.81). This is most likely due to the fact that pro-inflammatory cytokines are more prevalent in chronic kidney disease cases, which raises oxidative stress levels and ultimately triggers an inflammatory immunological response. The immune system damage that results may make people more vulnerable to bacterial and viral infections, which may be the main factor contributing to the increased risk of pulmonary inflammation. We discovered that among COVID-19-complicated chronic kidney disease cases, those under 70 had a greater rate of mortality than those 70 years of age or over. This is likely due to the increased likelihood of additional comorbidities in older individuals, such as COPD, hypertension, diabetes, and coronary heart disease. The particular disease linked to the elevated risk of death in chronic kidney disease cases is unclear, however these comorbidities will raise the risk of death connected with Covid-19 in the elderly.
According to Cheng et al.(54,55) prospective study, COVID-19's clinical course and mortality risk may be negatively impacted by prior chronic kidney disease. Additionally, a review that assessed the effect of the functions of the kidney on mortality and admission in 758 cases with SARS-CoV-2 infection, found that 8,5 % of infected cases had chronic renal disease and that 30,0 % of them had renal dysfunction upon admission (eGFR 60mL/min/1,73m2).20. According to the eGFR at admission, cases were divided into three groups: those with minimal renal impairment (eGFR >60mL/min/1,73m2), those with moderate renal impairment (eGFR 30–60mL/min/1,73m2), and those with severe renal impairment (eGFR 30mL/min/1,73m2). The complications such as sepsis (11,9 vs. 26,4 vs. 40,8 %, p0,001) and failure of respiratory system (35,4 vs. 72,2 vs. 62,0 %, p0,001) was higher among cases who had kidney dysfunction upon admission to the hospital.
Additionally, 19,7 % of cases had AKI at the time of admission, and those who had more severe renal dysfunction at that time were more likely to have their kidney function deteriorate while they were in the hospital (eGFR > 60 mL/min/1,73 m2 = 5,2 % vs. eGFR 30-60 mL/min/1,73 m2 = 31,8 % vs. eGFR 30 mL/min/1,73 m2 = 56. The survival possibility after twenty days was significantly lower in cases with eGFR30mL/min/1,73m2 (22,8 %) and eGFR 30-60mL/min/1,73m2 (27,2 %) compared to cases with an absence of substantial kidney failure during hospital admission (71,7 %).(54)
Another study discovered that cases with elevated SCr (32,4 %), those with prior chronic kidney disease (41,1 %), and those who developed AKI while in the hospital (15,9 %) had higher raw in-hospital rates of mortality than cases with normal SCr (5,8 %). A history of chronic kidney disease was present in 43,5 % of cases who had increased SCr values at the time of admission. According to a meta-analysis, individuals with chronic kidney disease had a threefold increased risk of developing severe disease. There are questions about this association22 because none of the studies specifically analyzed and took chronic kidney disease into account as a pre-existing condition or achieved statistical significance. Nevertheless, other research strengthened and confirmed the link between chronic kidney disease and a more severe COVID-19.(56)
Abrishami et al.(57) assessed the radiological and clinical characteristics of 43 adult chronic kidney disease cases with confirmed COVID-19 in a single-center investigation in Iran. These cases were discovered to have rates of mortality that were greater than those of the normal population and to be more susebtable to a more serious type of COVID-19.
The findings of this article are in agreement with those of Williamson et al.(58) who showed that COVID-19 cases with CKD have a considerably higher incidence of mortality. As the eGFR drops, the severity and mortality rise.
Patients with chronic kidney disease have higher overall readmission and rates of mortality, according to a multicenter cohort of COVID-19 cases who were admitted. In contrast to cases without chronic kidney disease, they demonstrate a significant rise in clinical mortality and rates of readmission in cases with any kind of CKD, with the exception of stage 5 CKD. The odds ratios in cases with chronic kidney disease stages 3a, 3b, 4, and KTX cases remain considerably higher compared to individuals without chronic kidney disease after all adjustments. Notably, the odd ratios among the chronic kidney disease groups did not show any correlation with the severity of the chronic kidney disease stage. They were unable to pinpoint the primary issue that caused the greater mortality and readmission rates. The authors were unable to identify any obvious patterns in vital signs or laboratory values at admission that would explain the elevated readmission and rates of mortality across chronic renal disease cohorts in our cohort, with the exception of lower diastolic blood pressure (chronic kidney disease stage 4) and significant variations in albumin and hemoglobin. The latter are likely directly related to chronic kidney disease stage rather than COVID-19. It is clear that chronic kidney disease as a whole is a risk factor for COVID-19 because there isn't a discernible component to account for the worse 12-week outcomes.(59)
This article's primary drawback is that it is a narrative review. A narrative review describes the findings of the included research in written paragraphs. They don't use the information from the summarised studies to perform any pooled analysis. This precludes pooled analysis and, hence, real objectivity. A narrative review functions as a collated source of the most widely held opinions at the time of publication. This can function as a useful approach to fully understand a body of evidence. It does not imply that the dominant theories are accurate as it does not fully examine the opposing hypothesis.
CONCLUSION
In Almaty, Kazakhstan, hospitalization outcomes for coronavirus patients with and without chronic kidney disease were assessed in this study. Analysis was done on the impact of comorbidities and sociodemographic characteristics on mortality.
1. The normal response of the adaptive and innate immune systems is impaired in people with chronic kidney disease.
2. While there are many ways to prevent and manage chronic kidney disease (CKD), coexisting medical diseases, particularly viruses that cause pandemics, might complicate matters. Thus, in order to prevent unplanned infectious disease outbreaks, a thorough disease management plan must be established.
3. This patient group is, therefore, more susceptible to persistent comorbid illnesses and viral infections.
4. Chronic kidney disease is mostly preventable and treatable, but multimorbid disorders, particularly viruses that cause pandemics, can change the course of events.
5. In order to prepare for unpredictable infectious disease outbreaks, a thorough disease management strategy must be established.
6. People who need maintenance dialysis may have a higher incidence of COVID-19 than individuals with CKD who do not need renal replacement treatment or those who have had a kidney, pancreas, or both kidneys transplanted.
7. People with chronic kidney disease and COVID-19 may experience a higher death rate than those with chronic kidney disease alone.
BIBLIOGRAPHIC REFERENCES
1. Garazhayeva L, Gaipov A, Zhakhina G, Kim V, Gusmanov A, Sakko Y, et al. WCN23-0601 Mortality of Patients With Ckd and its Risk Factors Admitted to Hospital With Covid-19 in Almaty City Kazakhstan Between 2020-2022. Kidney Int Rep [Internet]. 2023;8(3):S459–60. Available from: http://dx.doi.org/10.1016/j.ekir.2023.02.1031
2. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022 Apr;12(1):7–11. Doi/10.1016/j.kisu.2021.11.003
3. Ammirati AL. Chronic Kidney Disease. Rev Assoc Med Bras. 2020;66(suppl 1):s03–9.
4. Forbes A, Gallagher H. Chronic kidney disease in adults: assessment and management. Clin Med (Northfield Il). 2020 Mar;20(2):128–32.
5. Serhiyenko V, Holzmann K, Holota S, Derkach Z, Nersesyan A, Melnyk S, et al. An exploratory study of physiological and biochemical parameters to identify simple, robust and relevant biomarkers for therapeutic interventions for PTSD: study rationale, key elements of design and a context of war in Ukraine. Proc Shevchenko Sci Soc Med Sci. 2022;69(2).
6. Charles C, Ferris AH. Chronic Kidney Disease. Prim Care Clin Off Pract. 2020 Dec;47(4):585–95.
7. Maltsev D, Stefanyshyn V. The efficacy of combined immunotherapy with Propes and Inflamafertin in adult patients with genetic deficiency of the folate cycle and selective deficiency of <scp>NK</scp> and <scp>NKT</scp> cells. Immunology. 2022 Nov;167(3):443–50.
8. Maltsev, D., & Bokova S. Innovative Development of the Health Care Sector of the Future in the Conditions of Modern Challenges of the Covid-19 Coronavirus Infection in Ukraine. Futur Med. 2022 Mar;4–16.
9. Zhylin M, Makarenko S, Kolohryvova N, Bursa A, Tsekhmister Y. Risk Factors for Depressive Disorders after Coming through COVID-19 and Emotional Intelligence of the Individual. J Intellect Disabil - Diagnosis Treat. 2022 Oct;10(5):248–58.
10. Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, et al. COVID‐19 diagnosis and management: a comprehensive review. J Intern Med. 2020 Aug;288(2):192–206.
11. Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int J Environ Res Public Health. 2021 Mar;18(5):2621.
12. Ahorsu DK, Lin CY, Imani V, Saffari M, Griffiths MD, Pakpour AH. The Fear of COVID-19 Scale: Development and Initial Validation. Int J Ment Health Addict. 2022 Jun;20(3):1537–45.
13. Hussain N. The correlation between risk factors of COVID-19 and nervous system damage: Pakistan based Analysis. Futur Med. 2022 Sep;22–9.
14. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021 Jan;93(1):250-6.
15. Baig S. Change in physical and mental health due to aging: future perspective. Futur Med. 2023 Mar;13–23.
16. Yıldırım M, Akgül Ö, Geçer E. The Effect of COVID-19 Anxiety on General Health: the Role of COVID-19 Coping. Int J Ment Health Addict. 2022 Apr;20(2):1110–21.
17. Zhong BL, Luo W, Li HM, Zhang QQ, Liu XG, Li WT, et al. Knowledge, attitudes, and practices towards COVID-19 among Chinese residents during the rapid rise period of the COVID-19 outbreak: a quick online cross-sectional survey. Int J Biol Sci. 2020;16(10):1745–52.
18. Krasnova A. Vaccination of pregnant women against COVID-19 under martial law: a narrative review. Futur Med. 2022 Jun;4–12.
19. Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: An overview. J Chinese Med Assoc. 2020 Mar;83(3):217–20.
20. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J Adv Res. 2020 Jul;24:91–8.
21. Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, Lybeck M, et al. A Review of Persistent Post-COVID Syndrome (PPCS). Clin Rev Allergy Immunol. 2021 Feb;64(1):66–74.
22. Krishnan A, Hamilton JP, Alqahtani SA, Woreta TA. COVID-19: An overview and a clinical update. World J Clin Cases. 2021 Jan;9(1):8–23.
23. Hernandez Acosta RA, Esquer Garrigos Z, Marcelin JR, Vijayvargiya P. COVID-19 Pathogenesis and Clinical Manifestations. Infect Dis Clin North Am. 2022 Jun;36(2):231–49.
24. Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022 Mar;43(11):1157–72.
25. Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post‐COVID‐19 manifestations. Int J Clin Pract. 2021 Mar;75(3).
26. Huang X, Wei F, Hu L, Wen L, Chen K. Epidemiology and Clinical Characteristics of COVID-19. Arch Iran Med. 2020 Apr;23(4):268–71.
27. Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung. 2021 Apr;199(2):113–9.
28. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433.
29. Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, et al. Pregnancy and COVID-19. Physiol Rev. 2021 Jan;101(1):303–18.
30. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID‐19 based on current evidence. J Med Virol. 2020 Jun;92(6):548–51.
31. Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect. 2021 Apr;54(2):159–63.
32. Parnell D, Widdop P, Bond A, Wilson R. COVID-19, networks and sport. Manag Sport Leis. 2022 Mar;27(1–2):78–84.
33. Daniel SJ. Education and the COVID-19 pandemic. Prospects. 2020 Oct;49(1–2):91–6.
34. Sherman SM, Smith LE, Sim J, Amlôt R, Cutts M, Dasch H, et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum Vaccin Immunother. 2021 Jun;17(6):1612–21.
35. Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020 Apr;76:71–6.
36. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. 2021 Aug;38:101019.
37. Narayanan M, Setia S. Chronic Kidney Disease. In: The Perioperative Medicine Consult Handbook. Cham: Springer International Publishing; 2020. p. 301–5.
38. Iorember FM. Malnutrition in Chronic Kidney Disease. Front Pediatr. 2018 Jun;6.
39. Wang B, Li ZL, Zhang YL, Wen Y, Gao YM, Liu BC. Hypoxia and chronic kidney disease. eBioMedicine. 2022 Mar;77:103942.
40. Notarte KI, Catahay JA, Velasco JV, Pastrana A, Ver AT, Pangilinan FC, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. eClinicalMedicine. 2022 Nov;53:101624.
41. Ciotti M, Ciccozzi M, Terrinoni A, Jiang WC, Wang CB, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020 Aug;57(6):365–88.
42. Hebert SA, Ibrahim HN. Hypertension Management in Patients with Chronic Kidney Disease. Methodist Debakey Cardiovasc J. 2022 Sep;18(4):41–9.
43. Cao YL, Lin JH, Hammes HP, Zhang C. Flavonoids in Treatment of Chronic Kidney Disease. Molecules. 2022 Apr;27(7):2365.
44. Kramer H. Diet and Chronic Kidney Disease. Adv Nutr. 2019 Nov;10:S367–79.
45. Chung EYM, Palmer SC, Natale P, Krishnan A, Cooper TE, Saglimbene VM, et al. Incidence and Outcomes of COVID-19 in People With CKD: A Systematic Review and Meta-analysis. Am J Kidney Dis. 2021 Dec;78(6):804–15.
46. Pawar N, Tiwari V, Gupta A, Bhargava V, Malik M, Gupta A, et al. COVID-19 in CKD patients: Report from India. Indian J Nephrol. 2021;31(6):524.
47. Xu C, Zhang T, Zhu N, Han M. Characteristics of COVID-19 patients with preexisting CKD history. Int Urol Nephrol. 2021 Dec;53(12):2567–75.
48. Pakhchanian H, Raiker R, Mukherjee A, Khan A, Singh S, Chatterjee A. Outcomes of COVID-19 in CKD Patients. Clin J Am Soc Nephrol. 2021 May;16(5):785–6.
49. Akchurin O, Meza K, Biswas S, Greenbaum M, Licona-Freudenstein AP, Goyal P, et al. COVID-19 in Patients with CKD in New York City. Kidney360. 2021 Jan;2(1):63–70.
50. Leibler JH, Keogh SA, Jarquín E, Garcia-Trabanino R, Velázquez JJA, Pilarte DL, et al. COVID-19 and CKD: Employment, Food Security and Healthcare in El Salvador. Ann Glob Heal. 2022 Nov;88(1).
51. Bayesheva D, Boranbayeva R, Turdalina B, Fakhradiyev I, Saliev T, Tanabayeva S, et al. COVID-19 in the paediatric population of Kazakhstan. Paediatr Int Child Health. 2021 Jan;41(1):76–82.
52. Seilkhan A, Abdrassulova Z, Erkaebaeva M, Soltan R, Makhambetov M, Ydyrys A. Problems of distance education in Kazakhstan during the COVID-19 pandemic. World J Educ Technol Curr Issues. 2022 Mar;14(2):380–9.
53. Zhussupov B, Saliev T, Sarybayeva G, Altynbekov K, Tanabayeva S, Altynbekov S, et al. Analysis of COVID-19 pandemics in Kazakhstan. J Res Health Sci. 2021 May;21(2):e00512–e00512.
54. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97(5):829–38.
55. Uribarri A, Núñez-Gil IJ, Aparisi A, Becerra-Muñoz VM, Feltes G, Trabattoni D, et al. Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) Registry. J Nephrol. 2020 Aug;33(4):737–45.
56. Portolés J, Marques M, López-Sánchez P, de Valdenebro M, Muñez E, Serrano ML, et al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant. 2020 Aug;35(8):1353–61.
57. Abrishami A, Khalili N, Dalili N, Khaleghnejad Tabari R, Farjad R, Samavat S, et al. Clinical and Radiologic Characteristics of COVID-19 in Patients With CKD. Iran J Kidney Dis. 2020 Jul;14(4):267–77.
58. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020 Aug;584(7821):430–6.
59. Appelman B, Oppelaar JJ, Broeders L, Wiersinga WJ, Peters-Sengers H, Vogt L, et al. Mortality and readmission rates among hospitalized COVID-19 patients with varying stages of chronic kidney disease: a multicenter retrospective cohort. Sci Rep. 2022 Feb;12(1):2258.
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Garazhayeva Laura, Gaipov Abduzhappar.
Data curation: Kauysheva Almagul.
Formal analysis: Gaipov Abduzhappar.
Research: Kauysheva Almagul.
Methodology: Garazhayeva Laura.
Project management: Garazhayeva Laura.
Resources: Kauysheva Almagul.
Software: Gaipov Abduzhappar.
Supervision: Garazhayeva Laura.
Validation: Gaipov Abduzhappar, Kauysheva Almagul.
Visualization: Kauysheva Almagul.
Drafting - original draft: Garazhayeva Laura.
Writing - proofreading and editing: Gaipov Abduzhappar, Kauysheva Almagul.