doi: 10.56294/saludcyt2024.578
SYSTEMATIC REVIEW
Hyaluronan in lung, in plasma as pathogenic and prediction factor of acute respiratory distress syndrome: A systematic review
Hialuronano en pulmón, en plasma como factor patogénico y predictivo del síndrome de distrés respiratorio agudo: Una revisión sistemática
Evgen Dubrovskyi1
![]() *, Tetiana
Drevytska1
*, Tetiana
Drevytska1
![]() *, Victor Dosenko1
*, Victor Dosenko1
![]() *
*
1Department of General and Molecular Pathophysiology, Bogomoletz Institute of Physiology of the National Academy of Sciences of Ukraine. Kyiv, Ukraine.
Cite as: Dubrovskyi E, Drevytska T, Dosenko V. Hyaluronan in lung, in plasma as pathogenic and prediction factor of acute respiratory distress syndrome: A systematic review. Salud, Ciencia y Tecnología. 2024; 4:.578. https://doi.org/10.56294/saludcyt2024.578
Submitted: 14-02-2024 Revised: 19-05-2024 Accepted: 18-09-2024 Published: 19-09-2024
Editor: Dr.
William Castillo-González ![]()
Corresponding author: Evgen Dubrovskyi *
ABSTRACT
This investigation aims to study contemporary literature pertaining to the involvement of hyaluronate in the pathogenesis of diverse medical conditions, encompassing coronavirus-induced pulmonary injury, while also exploring its potential utility as a prognostic indicator for assessing the severity of COVID-19. This study conducted a comprehensive examination of hyaluronic acid’s multifaceted role in physiological processes and disease, with a specific focus on its implications in COVID-induced lung damage. The research provided an in-depth analysis of the intricate mechanisms and fundamental patterns governing these biological phenomena, elucidating essential interactions and pathways. Of particular significance in this investigation was the potential diagnostic utility of hyaluronic acid in assessing the severity of acute respiratory distress syndrome (ARDS), including COVID-19. Through a rigorous examination of hyaluronic acid concentration levels, researchers sought to assess its potential as an early prognostic indicator, thereby providing valuable insights for clinical diagnostics. Furthermore, the study explored the therapeutic prospects related to hyaluronic acid, emphasizing its involvement in various pathological processes. It suggested that targeting hyaluronic acid could represent a promising avenue for drug development, potentially leading to the creation of innovative pharmaceutical agents.
Keywords: Hyaluronan; Viral Pathogenicity; Acute Respiratory Distress Syndrome; Inflammatory Injury; Coronavirus.
RESUMEN
El objetivo de esta investigación es estudiar la bibliografía contemporánea relativa a la implicación del ácido hialurónico en la patogénesis de diversas afecciones médicas, incluida la lesión pulmonar inducida por coronavirus, al tiempo que se explora su posible utilidad como indicador pronóstico para evaluar la gravedad de la COVID-19. Este estudio llevó a cabo un examen exhaustivo del papel polifacético del ácido hialurónico en los procesos fisiológicos y las enfermedades, centrándose específicamente en sus implicaciones en el daño pulmonar inducido por COVID. La investigación proporcionó un análisis en profundidad de los intrincados mecanismos y patrones fundamentales que rigen estos fenómenos biológicos, elucidando interacciones y vías esenciales. De particular importancia en esta investigación fue la potencial utilidad diagnóstica del ácido hialurónico en la evaluación de la gravedad del síndrome de distrés respiratorio agudo (SDRA), incluyendo COVID-19. Mediante un examen riguroso de los niveles de concentración de ácido hialurónico, los investigadores trataron de evaluar su potencial como indicador de pronóstico precoz, aportando así valiosas ideas para el diagnóstico clínico. Además, el estudio exploró las perspectivas terapéuticas relacionadascon el ácido hialurónico, destacando su implicación en diversos procesos patológicos. Sugirió que el ácido hialurónico podría representar una vía prometedora para el desarrollo de fármacos, que podría conducir a la creación de agentes farmacéuticos innovadores.
Palabras clave: Hialuronano; Patogenicidad Viral; Síndrome de Distrés Respiratorio Agudo; Lesión Inflamatoria; Coronavirus.
INTRODUCTION
The novel coronavirus, SARS-CoV-2, which emerged in the second half of 2019 and causes COVID-19, was a major threat to life. The rapid spread of this disease has caused a significant burden of morbidity and mortality, especially among the elderly and at-risk groups. The catastrophic impact of the COVID-19 pandemic on health and the economy around the world has contributed to the rapid pace of studying the mechanisms of development of COVID-19 and its complications, the search for early markers of the development of complications, and drugs that prevent them. The global COVID-19 pandemic has led to a high demand for radiological examinations of the lungs. Patients with COVID-19 are known to have frequent computerised tomography (CT) scans. Specialists drew attention to an early visual symptom, which they called “frosted glass”. With the discovery of a specific diagnostic marker called “ground glass opacities”, there has been a great interest in studying and understanding the underlying cause of this phenomenon.(1,2,3)
U. Hellman et al.(4) from the Sweden Department of Public Health and Department of Laboratory, referring to autopsy findings in deceased COVID-19 patients whose lungs were filled with transparent liquid gel, that the macromolecule is pathologically associated with acute respiratory distress syndrome (ARDS) in severe form of COVID-19. Staining confirmed that hyaluronan obturates the alveoli with exudate and plugs, as well as in the thickened perialveolar interstitium. The hypothesis that the radiographic opacities seen on CT scans of COVID-19 patients, known as “ground glass opacities”, may be related to the accumulation of hyaluronic acid (HA), as in many other pathological conditions, has attracted the attention of researchers.(5) The glycosaminoglycan hyaluronan is present in virtually all tissues in the body and plays key roles in the regulation of tissue homeostasis, including vascular integrity.
Interstitial pulmonary edema is one of the leading symptoms of severe COVID-19 disease, directly related to acute respiratory failure and the need for oxygen support. Expanding on this suggestion, F. Zhao et al. at the University of Arizona also argue that HA is a potent water-binding agent that promotes interstitial and alveolar edema in ARDS, leading to histological findings of “hyaline membranes” seen in COVID-19 victims.(6) The HA molecule is highly hygroscopic and can absorb up to 1 thousand times its molecular weight in water, which can cause swelling.(7) Due to its high molecular weight and semi-flexible polymer chain, it forms a highly viscous gel-like liquid, conferring blocking properties and barrier functions in tissues. There are also a large number of HA-binding proteins that can involve HA in various biological processes.(8) High molecular weight HA predominates in most tissues under normal conditions, while fragmented low molecular weight HA polymers predominate in active inflammatory foci. After a comprehensive review of existing data regarding the role of hyaluronate in various pathological conditions, especially in lung pathology, research was initiated to investigate the connection between hyaluronate levels and the severity of COVID-19 in patients during the 2020-2021 epidemic in China. The experimental data obtained are consistent with numerous global studies and substantiate the hypothesis that hyaluronic acid serves as a significant prognostic marker for assessing the severity of COVID-19.(9)
The primary objective of this study is to comprehensively examine the role of hyaluronate in the pathogenesis of various diseases, including severe pulmonary forms of COVID-19. Additionally, it seeks to establish the level of hyaluronate in plasma as a prognostic factor for assessing the severity of COVID-19, thereby contributing to a better understanding of disease progression and potential clinical applications.
METHOD
To comprehensively consolidate existing knowledge on the relationship between hyaluronate and coronavirus, a two-stage approach was adopted, drawing inspiration from established analytical and bibliographic methodologies (Systematic Literature Review) recommended by S.K. Boell and D. Cecez-Kecmanovic.(10) This methodological framework was meticulously designed to minimise potential biases in data collection.
The research methodology began with an extensive keyword search using diverse terms like “hyaluronate”, “coronavirus”, and “COVID-19” across reputable sources, including PubMed, Web of Science, and Science Direct. This search encompassed articles from 2019 to 2023, along with foundational studies. In the initial stage, a broad search was conducted to identify relevant articles. The inclusion criteria focused on studies that investigated the biochemical and human aspects of hyaluronate’s role in coronavirus infections. Articles that explored non-hyaluronate interventions, were engineering-focused, or centered on theoretical developments without empirical findings were excluded. This rigorous screening process ensured the selection of studies directly related to the research objective. The second stage involved a meticulous screening of the identified articles. Duplicates were removed, and the remaining articles were assessed for relevance based on their titles and abstracts. Full-text reviews were then conducted for the shortlisted articles to ensure they met the inclusion criteria. This process resulted in the selection of 54 highly relevant research articles.
The selected articles underwent a systematic and comprehensive analysis, covering various research themes, focus areas, theoretical frameworks, and methodologies. This analysis provided a holistic view of the hyaluronate-coronavirus relationship in the literature. Key data points extracted from each study included the type of hyaluronate investigated, the context of its application, the outcomes observed, and any identified mechanisms of action. Data extraction was performed using a standardized form to maintain consistency and accuracy. The extracted data were then synthesized to identify common patterns and significant findings. This synthesis was structured to highlight the implications of hyaluronate in the progression and management of coronavirus infections, particularly COVID-19.
Throughout the study, efforts were made to ensure the reliability and validity of the findings. This included cross-checking data extraction and synthesis by multiple researchers and using established guidelines to minimize bias. The final analysis aimed to provide a comprehensive understanding of the role of hyaluronate in coronavirus infections, contributing valuable insights to the field of biomedical science and potential therapeutic approaches.
RESULTS
Results were refined to focus on biomedical science journal articles that considered both biochemical and human aspects of hyaluronate’s role in coronavirus infections, yielding 90 relevant articles. A meticulous screening process followed, applying inclusion and exclusion criteria precisely. Articles exploring hyaluronate in coronavirus contexts with empirical findings and human factors were included, while non-hyaluronate interventions, engineering-focused, or theoretical development articles were excluded. This approach identified 54 highly relevant research articles (figure 1). A systematic and comprehensive analysis of these selected papers covered research themes, focus areas, theoretical frameworks, and methodologies, offering a holistic view of the hyaluronate-coronavirus relationship in the literature.
Hyaluronate, also known as HA, plays a vital role in various physiological and pathological processes. It is synthesized by a diverse range of cell types, including fibroblasts, endothelial cells, alveolar cells, highlighting its widespread production across different contexts. HA is particularly noteworthy for its role in endothelial surface layer function, where it safeguards endothelial cells, regulates barrier permeability, and serves as a mechanosensory element essential for functions like nitric oxide production, vascular integrity, and vasodilation. HA possesses remarkable hydrophilic properties, allowing it to bind to water at a rate of up to 1000 times its weight. Consequently, HA can create highly viscous gels, which play a vital role in maintaining tissue balance and biomechanical stability. In the context of the lungs, HA is a dynamic molecule, and its physiological functions are closely linked to its molecular weight. Research indicates that low molecular weight HA, produced during tissue damage, can intensify inflammatory responses. Conversely, naturally occurring high molecular weight HA exerts a protective and anti-inflammatory influence.(9)
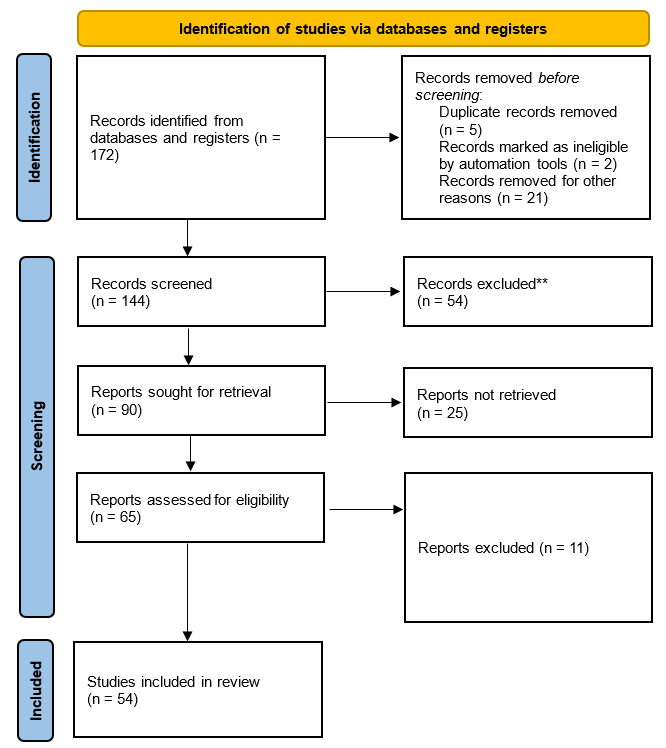
Figure 1. PRISMA flow diagram for new systematic reviews
|
Table 1. Normal concentration of hyaluronic acid in various tissues, organs, and fluids of the human body |
|
|
Tissues, organs, or fluids |
Concentration (mg/l) |
|
Umbilical |
4200 |
|
Synovial fluid |
2000-4000 |
|
Vitreous body |
150-340 |
|
Dermis |
200 |
|
Brain |
80 |
|
Thoracic lymph |
9-18 |
|
Urine |
0,1-0,5 |
|
Serum |
0,01-0,1 |
|
Source: S.R. Reeves(11) |
|
The significance of HA extends beyond maintaining homeostasis; it actively participates in numerous pathological processes, including those associated with aging, cancer, fibrosis, inflammation, diabetes, gastrointestinal diseases, and vascular conditions.(11,12) HA interacts with cellular receptors such as CD44, TLR4, HABP2, RHAMM, and LYVE1, which are present in neutrophils, macrophages, and endothelial cells. Activation of these signalling pathways triggers various processes, including NETs formation, macrophage activation, neutrophil degranulation, inflammation, thrombosis, lung injury, airway hyperactivation, vascular permeability, and microvascular endotheliopathy.(3)
The biological functions of HA are size-dependent. High molecular weight HA (> 1000 kDa), the physiologically available form, promotes cell survival and exhibits anti-inflammatory, anti-angiogenic, and immunosuppressive effects. Inter-α-trypsin inhibitor (IαI) and tumour necrosis factor-stimulated gene 6 (TSG-6) play crucial roles in modulating the impact of HA during lung inflammation. IαI, a proteoglycan generated in the liver and present in abundant quantities in the bloodstream, is also expressed by lung epithelial cells, fibroblasts, and airway smooth muscle cells. TSG-6, another significant hyaladherin, facilitates the chemical substitution of HA with IαI heavy chains, forming dense HC:HA complexes. These HC:HA structures extend the functional range of HA within the extracellular matrix and are identified in various inflammatory conditions, including asthma. Although HC:HA is recognised for its capacity to recruit immune cells in inflamed tissues, its precise influence on immune cell behaviour is still evolving. It is highly plausible that many of the roles conventionally ascribed to HA in animal models of inflammatory diseases are, in reality, orchestrated by HC:HA within the tissue.(13)
In contrast, low-molecular-weight HA (150–350 kDa), generated during inflammation, promotes cell migration and possesses proinflammatory and proangiogenic properties. Pro-inflammatory cytokines like TNFα, IL-1β, and lipopolysaccharides stimulate HA production in vitro by endothelial cells, dendritic cells, and fibroblasts.(14) Dysregulated HA synthesis and turnover in disease involve synthetic and catabolic enzymes such as HYAL1, HYAL2, and the KIAA1199 homolog TMEM2. Hyaluronan synthase genes (HAS1, HAS2, and HAS3), which are evolutionarily conserved despite being located on 19q13.41 chromosomes.(15) The expression profile of these HAS genes varies in different lung pathologies. For instance, increased levels of HAS1 and decreased levels of HAS2 are observed in patients with idiopathic pulmonary arterial hypertension, a condition associated with elevated total HA concentrations in the lungs.(16) In mouse models of asthma, HAS1 and HAS2 expression is upregulated in lung tissue. Additionally, HAS2-AS1, the natural antisense transcript of HAS2, plays a critical role in regulating HAS2 gene transcription, with implications in chromatin structure modulation.(17,18,19)
In the context of allergic reactions, HA acted to inhibit the interactions between IgE and FcɛRI receptors, as well as between FcɛRI receptors and PKCδ enzymes.(20) Additionally, HA interfered with the binding of CD44 receptors to PKCα enzymes, suggesting that HA primarily targeted CD44 receptors. PKCα and -δ enzymes played a role in increasing Rac1 activity and the expression of p47phox and p67phox. The presence of HA also inhibited the phosphorylation of PKCα and -δ. Rac1 activation was responsible for an increase in the production of reactive oxygen species (ROS), with NADPH oxidase being the primary source of these ROS. Blocking PKC activity prevented antigens from causing increased phosphorylation of ERK and p38 MAPK. These two pathways, along with ROS, were responsible for the secretion of β-hexosaminidase, histamine release, and the induction of chemokines.
HA was effective in suppressing the induction of chemokines, such as MIP-2 and Sprr-2a, in response to allergic stimuli. CD44 receptors played a crucial role in mediating the effects of antigens on the phosphorylation of ERK, p38 MAPK, ROS production, secretion of β-hexosaminidase, and histamine release. Importantly, G-protein-coupled receptors (GPCRs) did not influence the allergic response to antigens or affect the anti-allergic function of HA in this context. Elevated HA levels and its degradation products are evident in vivo models of chronic obstructive pulmonary disease (COPD), bleomycin-induced lung injury, fibrosis, allergic alveolitis, asthma, and idiopathic pulmonary arterial hypertension.(21) Furthermore, the accumulation of HA has been observed in models of allergic airway inflammation and lung damage caused by various factors, including ovalbumin, house dust mites, bleomycin, ozone, LPS, and staphylococcal enterotoxin B.(22) These observations underscore the relevance of HA in respiratory diseases and associated complications.
In the context of pulmonary hypertension, a significant discovery emerged from the study: elevated levels of hyaluronan were identified in patients with Group III PH.(23) These elevated levels correlated not only with increased activity of hyaluronan synthases (HAS) but also with the presence of hyaluronidases responsible for breaking down high molecular weight (HMW) hyaluronan into smaller fragments, which can contribute to the pathogenic processes of the disease. Notably, the study revealed compelling results indicating that treatment with 4MU, an inhibitor of hyaluronan synthesis that also affects HAS mRNA expression, had the potential to prevent the development of PH and treat established PH, particularly when associated with lung fibrosis. After lung injury, aberrant production of HA by adipocytes, epithelial cells, endothelial cells, and fibroblasts leads to excessive accumulation of HA in the airway lumen, thickening of the perialveolar interstitium and alveolar obstruction, thereby reducing lung compliance and ventilation. Small fragments of HA, formed by multiple mechanisms in the exudative phase of ARDS, actively participate in the signaling pathways of the cytokine release syndrome (CRS) or the so-called cytokine storm, manifesting as systemic immune-mediated processes, fever, and multiorgan dysfunction.(24) Small fragments of HA and oligosaccharides serve as Toll-like receptor (TLR) agonists to initiate inflammatory cascades that contribute to the recruitment and activation of inflammatory cells.
The team of authors from the Manchester Collaborative Centre for Inflammation Research also argue that although hyaluronate is an important component of the extracellular matrix of the lungs, its production and degradation must be carefully balanced.(8) In individuals with severe COVID-19, there is a notable presence of HA in their respiratory secretions compared to healthy individuals. A recent investigation identified a significant rise in HA levels, primarily of the low molecular weight form (LMW-HA), in the sputum of recently intubated patients. Additionally, this increase was accompanied by elevated levels of DNA originating from deceased or deteriorating cells. Treatment involving hyaluronidase and DNase revealed that these two substances play a role in the thick and viscous respiratory secretions characteristic of COVID-19-associated ARDS.(25) The researchers found an imbalance in hyaluronate production after the resolution of a severe lung influenza virus infection caused by hyaluronan synthase-2 from epithelial cells, endothelial cells, and fibroblasts.
Serum HA holds significant diagnostic importance and is closely linked to the pathogenesis of severe COVID-19. Evidence suggests that circulating HA fragments and serum hyaluronidase levels are strongly associated with organ failure and elevated inflammatory cytokine levels in COVID-19 patients.(26) Furthermore, serum HA serves as a predictor of the severity of COVID-19 disease. Collectively, these findings support the notion of a pathological role for GC in individuals with COVID-19. Considering HA not only as a pathogenic factor or an indicator of disease severity but also as a prospective target for the treatment of COVID-19 and potentially other diseases in the future presents a multifaceted opportunity.(27) This perspective implies that HA may hold therapeutic potential extending beyond its diagnostic relevance. Exploring HA as a treatment target could pave the way for innovative medical approaches. This broader view of HA’s role could be innovational how diseases, including COVID-19, are approached in terms of management.
DISCUSSION
The authors, who published their data in the Lancet journal on the results of studies of lung tissue of 38 deaths from COVID-19 in Italy in 2020, argued that diffuse alveolar damage is the predominant pattern of lung damage in patients with COVID-19.(28) This emphasises the importance of preventing or at least accurately predicting the development of such disease manifestations. A potential solution to this challenge could involve screening for hyaluronate levels. Numerous researchers, such as C.Y. Lin et al. have already proposed a strong association between HA and severe cases of COVID-19, particularly those characterized by a “cytokine storm” featuring elevated levels of inflammatory cytokines like IL-1β, IL-6, and TNFα.(29) This link is rooted in the fact that these cytokines are potent stimulators of HA synthase 2 (HAS2) in various cell types such as endothelial cells, pulmonary alveolar epithelial cells, and fibroblasts. Consequently, it aligns with the concept that a state of heightened inflammation leads to the production and build-up of HA in the alveolar spaces of individuals afflicted with severe COVID-19. The presence of abundant HA in the lungs, particularly within the alveolar spaces, in individuals who succumbed to the most severe form of COVID-19 suggests a potential pathogenetic mechanism contributing to the hypoxemia and respiratory failure observed in these critically ill patients. This underscores the importance of exploring the role of HA in the context of severe COVID-19 and its potential implications for patient management and treatment strategies.
Confirmation of abundant ground-glass opacities in the lungs, particularly in the alveolar spaces of individuals who died from the most severe form of COVID-19, thus indicates a potential pathogenetic mechanism for the hypoxemia and respiratory insufficiency observed in these critically ill patients. U. Hellman et al., hailing from the Sweden Department of Public Health and Department of Laboratory, were among the early pioneers in suggesting a pathological link between pulmonary hyaline membrane disease (PHMD) and acute respiratory distress syndrome (ARDS) based on autopsy findings in deceased COVID-19 patients.(30) In summary, Ur. Hellman and his team proposed a compelling association between PHMD and ARDS in COVID-19 cases, with hyaluronan playing a pivotal role in the pathological processes observed. This hypothesis has ignited further research into the links between HA accumulation and ground glass opacities seen in COVID-19 patients, shedding light on the potential mechanisms underlying the disease’s severity.
The genetic study conducted by S. Andonegui-Elguera et al. from Mexico is remarkably noteworthy, as it demonstrates molecular findings in bronchoalveolar cells regarding the activation of genes encoding proteins involved in the metabolism of hyaluronan, glycosaminoglycans, aminoglycans, and mucopolysaccharides.(31) This confirms the notion that complex polysaccharides indeed play a crucial role in the development of severe forms of interstitial pulmonary edema. A. Khoor, in his proposal, suggests that the activation of genes responsible for the enzymes involved in hyaluronic acid and glycosaminoglycan metabolism within lung cells may be a fundamental factor contributing to the development of severe interstitial lung damage observed in COVID-19 cases.(32) This activation could potentially underlie the distinctive pathological changes seen in the lungs of affected individuals. It is undeniable that both U. Hellman et al. and M.J. Kratochvil et al. have found substantial amounts of hyaluronic acid in the lung tissues, bronchoalveolar lavage, and sputum of individuals suffering from acute respiratory distress syndrome (ARDS) in the context of COVID-19.(30,28) It’s worth highlighting that serum hyaluronic acid plays a significant role in the pathogenesis of severe COVID-19. This assertion is substantiated by the research carried out by Queisser et al., whose findings demonstrate a strong connection between circulating hyaluronic acid fragments (HA) and hyaluronidase in the serum and the presence of organ dysfunction and heightened levels of inflammatory cytokines in COVID-19 patients.(33)
According to G.B. Johnson et al., there is substantial evidence indicating that SARS-CoV-2 infection leads to the degradation of the endothelial glycocalyx.(34) Moreover, the cytokine profile characteristic of COVID-19 prompts abnormal synthesis and degradation of the glycocalyx in pulmonary endothelial cells. When human pulmonary microvascular endothelial cells are exposed to plasma from COVID-19 patients, this contributes to the release of the glycocalyx into the surrounding environment and increases hyaluronidase activity. The degradation products of hyaluronic acid, in this context, serve as intrinsic danger signals capable of intensifying pro-inflammatory responses, both in laboratory experiments and in mouse models of the disease. Taking into consideration the accumulated data, it becomes evident why certain scientific groups, such as Ming Ding et al. from Southeast University, Nanjing, China, propose the use of serum HA levels as a predictor of the severity of COVID-19.(35)
The debate still remains regarding the primary step in the development of the pathological cascade. There is evidence suggesting that in COVID-19-associated acute respiratory distress syndrome (ARDS), syndecan-1 increases the level of IL-6, while hyaluronic acid directly activates NRP1, a co-receptor of activated VEGFA (Vascular endothelial growth factor A), which is associated with pulmonary vascular hyperpermeability. Additionally, it interacts with VCAN (Vascular cell adhesion molecule), a proteoglycan that plays a crucial role in chemokine communication and upregulation.(36) Based on the analysis of acquired data and existing knowledge, the future application of hyaluronate level determination as a prognostic marker in diseases associated with ARDS appears promising.(28,27,38,39,40) When combined with other indicators of disease severity, the hyaluronate level offers the potential to predict the overall disease severity. While the determinants of disease severity are multifaceted and encompass various parameters, early-stage assessment of serum hyaluronic acid levels can provide valuable insights into the subsequent disease progression.
Understanding the link between hyaluronic acid and ARDS has led to the search for therapeutic models to reduce HA levels. R.J. McKallip et al. tested the positive effect of 4-methylumbelliferol [4-MU], better known as the drug hymecromone, which is used to treat biliary spasms, in animals.(41) Another team of researchers J.I. Rosser et al. has already tested the effect of 4-methylumbelliferol on hyaluronic acid synthesis in healthy volunteers and demonstrated that sputum levels of HA were significantly reduced on the first day of taking hymecromone.(21)
Our review’s strengths lie in its systematic approach to including only empirical studies that investigated the role of HA in coronavirus infections, ensuring a focused and relevant selection of research. However, several limitations should be noted. The variability in study designs, sample sizes, and methodologies across the included studies could introduce heterogeneity in the findings. Additionally, the predominance of in vitro and animal studies may limit the direct applicability of some findings to human clinical practice.
Our findings align with previous studies that have highlighted the role of HA in modulating immune responses and inflammation. For instance, the study by C.Y. Lin et al.(29) demonstrated a strong association between HA levels and severe COVID-19 outcomes, emphasizing the role of inflammatory cytokines such as IL-1β, IL-6, and TNFα in stimulating HA synthesis. Similarly, the findings by U. Hellman et al.(30) established a link between hyaline membrane disease and ARDS in COVID-19, further supporting the critical role of HA in lung pathology. The evidence suggests that HA levels could serve as a valuable biomarker for predicting the severity of COVID-19. Elevated serum HA and hyaluronidase levels are significantly associated with organ failure and elevated inflammatory cytokines in COVID-19 patients.(26,33) This underscores the potential of HA as a diagnostic marker and a therapeutic target. The ability to measure HA levels could enhance the prediction of disease progression and inform treatment strategies, potentially guiding the development of novel therapeutic interventions targeting HA metabolism.
Our review highlights several areas for future research. There is a need for more robust clinical studies to validate the use of HA as a prognostic marker in COVID-19 and other respiratory diseases. Additionally, further research is required to elucidate the mechanisms by which HA contributes to disease pathogenesis, particularly in the context of cytokine storms and ARDS. Investigating the therapeutic potential of HA modulators, such as 4-methylumbelliferol (4-MU), could offer new avenues for treating severe respiratory conditions associated with viral infections.(41) In conclusion, our systematic review provides compelling evidence supporting the role of HA in the pathogenesis of severe COVID-19. The findings indicate that HA not only serves as a diagnostic biomarker but also holds promise as a therapeutic target. Future research should aim to explore these possibilities further, potentially leading to innovative treatments that could mitigate the impact of severe viral infections on respiratory health.
CONCLUSIONS
In summary, the extensive literature analysis has shed light on the often-underestimated role of hyaluronan in plasma as an early predictor of the severity of COVID-19, with implications extending to conditions secondary to COVID-19, such as allergies, pulmonary fibrosis, and COPD. Hyaluronan’s unique properties make it a significant contributor to immune modulation, impacting homeostasis, inflammation, and tissue repair. When maintained in equilibrium, hyaluronan serves a protective function. However, disruptions in this balance can lead to complications, not only in COVID-19 but also in conditions exacerbated by the virus. This comprehensive review delves into hyaluronan’s multifaceted role as an early predictor of COVID-19 severity and explores its molecular intricacies in the context of allergies, pulmonary fibrosis, and COPD, all of which can be exacerbated by COVID-19. Recent findings suggest that elevated levels of hyaluronan in plasma may correlate with the severity of COVID-19, including the risk of developing acute respiratory distress syndrome (ARDS), which can further complicate pre-existing conditions such as allergies and COPD. Additionally, hyaluronan’s involvement in tissue repair and inflammation makes it relevant in understanding and managing post-COVID-19 pulmonary fibrosis. These insights align with global studies and emphasise hyaluronan’s potential as a valuable prognostic marker not only for assessing the seriousness of COVID-19 but also for understanding the complexities of respiratory conditions that may be exacerbated or triggered by the virus. In conclusion, this literature review underscores the multifaceted role of hyaluronan in predicting the severity of COVID-19 and its interconnectedness with allergies, pulmonary fibrosis, and COPD in the context of the virus. These insights may pave the way for further research and the development of strategies to use hyaluronan levels in plasma as an early predictor, aiding in the timely management and intervention of complex respiratory conditions linked to COVID-19.
REFERENCES
1. Mostovyi S (2023). Comparative analysis of the glomerular filtration rate effect on the course of COVID-19 in patients with coronary heart disease with and without concomitant coronavirus disease. Int J Med Med Res. 9(1), 15-23. https://doi.org/10.61751/ijmmr.2413-6077.2023.1.15
2. Bakalets O, Dzyha S, Behosh N (2023). Functional diagnostics of the respiratory system in patients with Long COVID. Bull Med Biol Res. 16(2), 60-66. https://doi.org/10.61751/bmbr.2706-6290.2023.2.60
3. Dobrovanov O, Dmytriiev D, Prochotsky A, Vidiscak M, Furkova K (2023). Chronic pain in post-COVID syndrome. Bratisl Med J. 124(2), 97-103. https://doi.org/10.4149/BLL_2023_014
4. Hellman U, Karlsson MG, Engström-Laurent A, Cajander S, Dorofte L, Ahlm C, Laurent C, Blomberg A. (2020). Presence of hyaluronan in lung alveoli in severe Covid-19: An opening for new treatment options? J Bio Chemist. 295(45), 15418-15422. https://doi.org/10.1074/jbc.ac120.015967
5. Ismailov I, Kalmatov R, Abdurakhmanov B, Mirza AM, Chaurasia JK (2024). Role of reactive oxygen species in the pathogenesis of bronchial asthma and obstructive pulmonary diseases: systematic review. Adv Life Sci. 11(2), 286-295. https://doi.org/10.62940/als.v11i2.2380
6. Zhao F, Barber C, Sammani S, Wan L, Miller B, Furenlid L, Li Z, Gotur D, Barrios R, Woolfenden J, Martin D, Liu Z. (2022). Use of radiolabeled hyaluronic acid for preclinical assessment of inflammatory injury and acute respiratory distress syndrome. Nucl Med Bio. 114, 86-98. https://doi.org/10.1016%2Fj.nucmedbio.2022.10.002
7. Topchubaeva ET, Imetova ZB, Turusbekova AK, Abdurahmanov BO, Kalmatov RK (2020). Respiratory tract disorders associated with changes of the mucous membrane in workers often exposed to pathological and toxic factors. J Environ Treat Tech. 8(4), 1581-1585. https://doi.org/10.47277/JETT/1585
8. Belov GV, Sultanmuratov MT, Kalmatov RK, Dzholdubaev YD, Akmatov KT (2005). Response to exercise of surfactant system of the lungs and lipid peroxidation in rats adapted to low and high altitude climate. Vopr Kurortol Fizioter Lech Fiz Kult. 3, 34-35. https://pubmed.ncbi.nlm.nih.gov/16060282/
9. Bongiovanni A, Parisi GF, Scuderi MG, Licari A, Brambilla I, Marseglia GL, Leonardi S (2019). Gastroesophageal reflux and respiratory diseases: does a real link exist? Minerva Pediatr. 71(6), 515-523. https://doi.org/10.23736/S0026-4946.19.05531-2
10. Boell SK, Cecez-Kecmanovic D. (2016). On being ‘systematic’ in literature reviews. In: Formulating Research Methods for Information Systems (pp. 48-78). London: Palgrave Macmillan. https://doi.org/10.1057/jit.2014.26
11. Reeves SR, Barrow KA, Rich LM, White MP, Shubin NJ, Chan CK, Kang I, Ziegler SF, Piliponsky AM, Wight TN, Debley JS. (2020). Respiratory syncytial virus infection of human lung fibroblasts induces a hyaluronan-enriched extracellular matrix that binds mast cells and enhances expression of mast cell proteases. Front Immunol. 10, 3159. https://doi.org/10.3389/fimmu.2019.03159
12. Kovalchuk VP, Nazarchuk OA, Burkot VM, Fomina NS, Prokopchuk ZM, Dobrovanov O (2021). Biofilm forming activity of non-fermenting gram-negative bacteria. Wiad Lek. 74(2), 252-256. https://doi.org/10.36740/wlek202102114
13. Hällgren R, Samuelsson T, Laurnet T, Modig J. (1989). Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Diseas. 139(3), 682-687. https://doi.org/10.1164/ajrccm/139.3.682
14. Laurent TC, Laurent UBG, Fraser JRE. (1996). The structure and function of hyaluronan: An overview. Immun Cell Bio. 74(2), a1-a7. https://doi.org/10.1038/icb.1996.32
15. Day AJ, Prestwich GD. (2002). Hyaluronan-binding proteins: tying up the giant. J Bio Chemis. 277(7) 4585-4588. https://doi.org/10.1074/jbc.r100036200
16. Csoka AB, Frost G, Stern R. (2001). The six hyaluronidase-like genes in the human and mouse genomes. Matrix Bio. 20(8), 499-508. https://doi.org/10.1016/s0945-053x(01)00172-x
17. Donlan AN, Sutherland TE, Marie C, Preissner S, Bradley BT, Carpenter RM, Sturek JM, Ma JZ, Moreau GB, Donowitz JR, Buck GA, Serrano MG, Burgess SL, Abhyankar MM, Mura C, Bourne PE, Preissner R, Young MK, Lyons GR, Loomba JJ, Ratcliffe SJ, Poulter MD, Mathers AJ, Day AJ, Mann BJ, Allen JE, Petri WA. (2021). IL-13 is a driver of COVID-19 severity. JCI Insigh. 6(15), e150107. https://doi.org/10.1101%2F2020.06.18.20134353
18. Vigetti D, Deleonibus S, Moretto P, Bowen T, Fischer JW, Grandoch M, Oberhuber A, Love D, Hanover J, Cinquetti R, Karousou E, Viola M, D’angelo ML, Hascall V, de Luca G, Passi A. (2014). Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcNAcylation. J Bio Chemis. 289(42), 28816-28826. https://doi.org/10.1074/jbc.m114.597401
19. Song C, Chai Q, Danielsen C, Hjorth P, Nyengaard JR, Ledet T, Yamaguchi Y, Rasmussen L, Wogensen L. (2005). Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circulat Res. 96(5), 583-591. https://doi.org/10.1161/01.res.0000158963.37132.8b
20. Kim Y, Lee YS, Hahn JH, Choe J, Kwon HJ, Ro JY, Jeoung D. (2008). Hyaluronic acid targets CD44 and inhibits FcɛRI signaling involving PKCδ, Rac1, ROS, and MAPK to exert anti-allergic effect. Molecul Immun. 45(9), 2537-2547. https://doi.org/10.1016/j.molimm.2008.01.008
21. Rosser JI, Nagy N, Goel R, Kaber G, Demirdjian S, Saxena J, Bollyky JB, Frymoyer AR, Pacheco-Navarro AE, Burgener EB, Rajadas J, Wang Z, Arbach O, Dunn CE, Kalinowski A, Milla CE, Bollyky PL. (2022). Oral hymecromone decreases hyaluronan in human study participants. J Clinic Invest. 132(9), e157983. https://doi.org/10.1172%2FJCI157983
22. Queisser KA, Mellema RA, Middleton EA, Portier I, Manne BK, Denorme F, Beswick EJ, Rondina MT, Campbell RA, Petrey AC. (2021). COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction. JCI Insigh. 6(17), e147472. https://doi.org/10.1172/jci.insight.147472
23. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo Antinori PS, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. (2020). Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect Diseas. 20(10), 1135-1140. https://doi.org/10.1016/s1473-3099(20)30434-5
24. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Chen L, Li J, Wang X, Wang F, Liu L, Zhang S, Zhang Z. (2020). The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. https://doi.org/10.1101/2020.02.23.20026690
25. Borrmann M, Brandes F, Kirchner B, Klein M, Billaud JN, Reithmair M, Rehm Schelling G, Pfaffl M, Meidert AS. (2023). Extensive blood transcriptome analysis reveals cellular signaling networks activated by circulating glycocalyx components reflecting vascular injury in COVID-19. Front Immun. 14, 1129766. https://doi.org/10.3389/fimmu.2023.1129766
26. Yang S, Tong Y, Chen L, Yu W. (2022). Human Identical Sequences, hyaluronan, and hymecromone – The new mechanism and management of COVID-19. Molecul Biomed. 3, 15. https://doi.org/10.1186/s43556-022-00077-0
27. Huang JJ, Wang CW, Liu Y, Zhang YY, Yang NB, Yu YC, Jiang Q, Song QF, Qian GQ. (2023). Role of the extracellular matrix in COVID-19. World J Clinic Cases. 11(1), 73. https://doi.org/10.12998/wjcc.v11.i1.73
28. Kratochvil MJ, Kaber G, Demirdjian S, Cai PC, Burgener EB, Nagy N, Barlow GL, Popescu M, Nicolls MR, Ozawa MG, Regula DP, Pacheco-Navarro AE, Yang S, de Jesus Perez VA, Karmouty-Quintana H, Peters AM, Zhao B, Buja ML, Johnson PY, Vernon RB, Bollyky PL. (2022). Biochemical, biophysical, and immunological characterization of respiratory secretions in severe SARS-CoV-2 infections. JCI Insigh. 7(12), e152629. https://doi.org/10.1172/jci.insight.152629
29. Lin CY, Kolliopoulos C, Huang CH, Tenhunen J, Heldin CH, Chen YH, Heldin P. (2019). High levels of serum hyaluronan is an early predictor of dengue warning signs and perturbs vascular integrity. EBioMed. 48, 425-441. https://doi.org/10.1016/j.ebiom.2019.09.014
30. Hellman U, Rosendal E, Lehrstrand J, Henriksson J, Björsell T, Hahn M, Österberg B, Dorofte L, Nilsson E, Forsell M, Smed-Sörensen A, Lenman A. (2023). Hyaluronan in COVID-19 morbidity, a bedside-to-bench approach to understand mechanisms and long-term consequences of hyaluronan. https://www.medrxiv.org/content/10.1101/2023.02.10.23285332v2
31. Andonegui-Elguera S, Taniguchi-Ponciano K, Gonzalez-Bonilla C, Torres J, Mayani H, Herrera L, Pena-Martínez E, Silva-Román G, Vela-Patiño S, Ferreira-Hermosillo A, Ramirez-Renteria C, Carvente-Garcia R, Mata-Lozano C, Marrero-Rodríguez D, Mercado M. (2020). Molecular alterations prompted by SARS-CoV-2 infection: induction of hyaluronan, glycosaminoglycan and mucopolysaccharide metabolism. Archiv Med Res. 51(7), 645-653. https://doi.org/10.1016/j.arcmed.2020.06.011
32. Khoor A. (2008). Idiopathic interstitial pneumonias. London: Elsevier. https://mayoclinic.elsevierpure.com/en/publications/idiopathic-interstitial-pneumonias
33. Queisser KA, Mellema RA, Petrey AC. (2021). Hyaluronan and its receptors as regulatory molecules of the endothelial interface. J Histochem Cytochem. 69(1), 25-34. https://doi.org/10.1369/0022155420954296
34. Johnson GB, Brunn GJ, Platt JL. (2004). Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immun. 172(1), 20-24. https://doi.org/10.4049/jimmunol.172.1.20
35. Ding M, Zhang Q, Li Q, Wu T, Huang Y. (2020). Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respir Med. 167, 105981. https://doi.org/10.1016%2Fj.rmed.2020.105981
36. Barnes HW, Demirdjian S, Haddock NL, Kaber G, Martinez H, Nagy N, Karmouty-Quintana H, Bollyky PL. (2023). Hyaluronan in the pathogenesis of acute and post-acute COVID-19 infection. Matrix Bio. 116, 49-66. https://doi.org/10.1016%2Fj.matbio.2023.02.001
37. Rodén L, Campbell P, Fraser R, Laurent T, Pertoft H, Thompson J. (2007). Enzymic pathways of hyaluronan catabolism. In: The Biology of Hyaluronan: Ciba Foundation Symposium 143. https://doi.org/10.1002/9780470513774.ch5
38. Baranova NS, Nilebäck E, Haller FM, Briggs DC, Svedhem S, Day AJ, Richter RP. (2011). The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J Bio Chemis. 286(29), 25675-25686. https://doi.org/10.1074/jbc.m111.247395
39. Collum SD, Chen NY, Hernandez AM, Hanmandlu A, Sweeney H, Mertens T, Weng T, Luo F, Molino J, Davies J, Horan IP. (2017). Inhibition of hyaluronan synthesis attenuates pulmonary hypertension associated with lung fibrosis. British J Pharmac. 174(19), 3284-3301. https://doi.org/10.1111%2Fbph.13947
40. Albtoush N, Petrey AC. (2022). The role of hyaluronan synthesis and degradation in the critical respiratory illness COVID-19. Am J Phys-Cell Physiol. 322(6), 1037-1046. https://doi.org/10.1152/ajpcell.00071.2022
41. Alvarado MAG. Gentrification and Community Development: An analysis of the main lines of research. Gentrification 2023;1:2–2. https://doi.org/10.62486/gen20232.
42. Auza-Santivañez JC, Apaza-Huanca B, Diaz-Guerrero JL, Vallejos-Rejas DRE, Zelaya-Espinoza Y, Vargas-Gallego I, et al. Relevance of ultrasound detection and assessment of vascular calcifications in chronic kidney disease. Multidisciplinar (Montevideo) 2024;2:77–77. https://doi.org/10.62486/agmu202477.
43. Ayala DP, Falero DML, Pita MM, González IC, Silva JW. Ozone therapy in periodontal disease in type 2 diabetic patients. Odontologia (Montevideo) 2024;2:120–120. https://doi.org/10.62486/agodonto2024120.
44. Benavidez AMV, Ozeta RJN, Davila CAC, Hernandez OH, Mogollon JAY. Conceptual maps using Cmap Tools and student learning at a National Intercultural University Fabiola Salazar Leguía of Bagua. Multidisciplinar (Montevideo) 2024;2:107–107. https://doi.org/10.62486/agmu2024107.
45. Cano CAG. Education, urbanism, and gentrification: convergence of issues and solutions. Gentrification 2023;1:1–1. https://doi.org/10.62486/gen20231.
46. Cardozo GT. Community development promoted by policies: an analysis from the perspective of gentrification. Gentrification 2023;1:3–3. https://doi.org/10.62486/gen20233.
47. Dinkar AK, Haque MA, Choudhary AK. Enhancing IoT Data Analysis with Machine Learning: A Comprehensive Overview. LatIA 2024;2:9–9. https://doi.org/10.62486/latia20249.
48. Espinoza GYG. Transforming the Salitre campus into a smart campus: proposal of smart initiatives for the Gerardo Barrios University of El Salvador. LatIA 2024;2:102–102. https://doi.org/10.62486/latia2024102.
49. Galván LNO, Ayala DP, Lozano IM, Falero DML, Silva JW. Breastfeeding, Oral Habits, and Malocclusions in Children Aged 3 to 6 Years. Odontologia (Montevideo) 2024;2:101–101. https://odonto.ageditor.uy/index.php/odonto/article/view/101
50. García EA, Curbelo ML, Iglesias MSS, Falero DML, Silva JW. Oral lesions associated with the use and care of dentures in the elderly. Odontologia (Montevideo) 2024;2:100–100. https://doi.org/10.62486/agodonto2024100.
51. González MS, Pérez AG. Proposal of actions to improve accessibility at the Hotel Las Yagrumas, Artemisa. Management (Montevideo) 2024;2:25–25. https://doi.org/10.62486/agma202425.
52. Hernández-Lugo M de la C. Artificial Intelligence as a tool for analysis in Social Sciences: methods and applications. LatIA 2024;2:11–11. https://doi.org/10.62486/latia202411.
53. Iyengar MS, Venkatesh R. A Brief Report on Building Customer Loyalty in Luxury hotels: A Universal Approach. Management (Montevideo) 2024;2:32–32. https://doi.org/10.62486/agma202432.
54. Iyengar MS, Venkatesh R. Customer preferences while booking accommodation in hotels: Customer Behaviour and Hotel Strategies. Management (Montevideo) 2024;2:31–31. https://doi.org/10.62486/agma202431.
55. León MP. The impact of gentrification policies on urban development. Gentrification 2023;1:4–4. https://doi.org/10.62486/gen20234.
56. Lozano IM, Molina YG, Santos IF, Galván LNO, Pérez AP, Becerra CEC. Behavior of Denture Stomatitis in Adults Over 45 Years of Age. Odontologia (Montevideo) 2024;2:102–102. https://doi.org/10.62486/agodonto2024102.
57. Macedo GC, Auza-Santivañez JC, Rejas DREV, Sarmiento RAQ, Canaviri JJF, Laime LHS. Giant multiloculated omental cyst in a pediatric patient. Case report and literature review. Multidisciplinar (Montevideo) 2024;2:88–88. https://doi.org/10.62486/agmu202488.
58. Martinez AME, Warnes CD. English language skills and their influence on the academic performance of high school students in the public schools of Cereté – Córdoba. Multidisciplinar (Montevideo) 2024;2:108–108. https://doi.org/10.62486/agmu2024108.
59. Ramirez GAM, Murillo MYR, Valderrama PJC, Patiño ML, Mora YJR. Analysis of the strategic plan for the Acuña Ventures SAS company in Yopal city, Colombia. Management (Montevideo) 2024;2:29–29. https://doi.org/10.62486/agma202429.
60. Sonal D, Mishra K, Haque A, Uddin F. A Practical Approach to Increase Crop Production Using Wireless Sensor Technology. LatIA 2024;2:10–10. https://doi.org/10.62486/latia202410.
61. Vargas OLT, Agredo IAR. Active packaging technology: cassava starch/orange essential oil for antimicrobial food packaging. Multidisciplinar (Montevideo) 2024;2:102–102. https://doi.org/10.62486/agmu2024102.
62. McKallip RJ, Ban H, Uchakina ON. (2015). Treatment with the hyaluronic acid synthesis inhibitor 4-methylumbelliferone suppresses LPS-induced lung inflammation. Inflam. 38, 1250-1259. https://doi.org/10.1007/s10753-014-0092-y
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Evgen Dubrovskyi, Victor Dosenko.
Data curation: Evgen Dubrovskyi.
Formal analysis: Victor Dosenko.
Research: Evgen Dubrovskyi, Tetiana Drevytska.
Methodology: Tetiana Drevytska, Victor Dosenko.
Project management: Tetiana Drevytska, Victor Dosenko.
Resources: Tetiana Drevytska.
Validation: Evgen Dubrovskyi.
Drafting - original draft: Tetiana Drevytska.
Writing - proofreading and editing: Evgen Dubrovskyi, Victor Dosenko.