doi: 10.56294/saludcyt20241353
REVIEW
Pregnancy-Related Factors and Prediction of Peripartum Stroke
Factores relacionados con el embarazo y predicción del ictus periparto
Zhanar Kypshakbayeva1 ![]() *, Almagul Kurmanova2
*, Almagul Kurmanova2 ![]() *, Gaukhar Kurmanova3
*, Gaukhar Kurmanova3 ![]() *, Damilya Salimbayeva4
*, Damilya Salimbayeva4 ![]() *, Madina Khalmyrsayeva, Aizhan Turekhanova5
*, Madina Khalmyrsayeva, Aizhan Turekhanova5 ![]() *
*
1Kazakhstan’s Medical University “KSPH” LLP, Almaty, Kazakhstan.
2Department of Fundamental Medicine, Al Farabi Kazakh National University, Almaty, Kazakhstan.
3Al Farabi Kazakh National University, Almaty, Kazakhstan.
4Department of Science and Strategic Development, Scientific Center of Obstetrics, Gynecology, and Perinatology, Almaty, Kazakhstan.
5Al Farabi Kazakh National University, Almaty, Kazakhstan.
Cite as: Kypshakbayeva Z, Kurmanova A, Kurmanova G, Salimbayeva D, Khalmyrsayeva M, Turekhanova A. Pregnancy-Related Factors and Prediction of Peripartum Stroke. Salud, Ciencia y Tecnología. 2024; 4:1353. https://doi.org/10.56294/saludcyt20241353
Submitted: 05-03-2024 Revised: 29-05-2024 Accepted: 06-08-2024 Published: 07-08-2024
Editor: Dr.
William Castillo-González ![]()
ABSTRACT
Aim: to demonstrate the prediction tools, risk factors, and predisposing factors of peripartum stroke and its impact on the fetus and the mother.
Method: in our review, we involved English studies from common databases such as Google Scholar, Pubmed/MEDLINE, Scopus, Web of Science, and the Cochrane Library with the keywords “prepartum,” “postpartum,” and “peripartum,” combined with keywords involving “maternal stroke,’’ and ‘’detection.” The end date for this review is September 2023.
Scientific novelty: there are several studies that tried to determine the incidence, risk factors, management, and complications of maternal stroke; however, there are studies that attempted to identify an accurate prediction tool for peripartum stroke. In our article, we tried to identify an accurate prediction tool for peripartum stroke.
Conclusion: our understanding of maternal stroke still has several limitations. Although risk indicators have been established, there are currently no techniques available to determine which females are more likely to experience postpartum strokes and need closer observation. The risk of a subsequent maternal stroke has not been clearly assessed, which limits the advice that clinicians can give patients.
Keywords: Reversible Cerebral Vasoconstriction Syndrome; Maternal Stroke; Cerebral Venous Thrombosis; Intracranial Hemorrhage; Preeclampsia.
RESUMEN
Objetivo: demostrar las herramientas de predicción, los factores de riesgo y los factores predisponentes del ictus periparto y su impacto en el feto y la madre.
Método: en nuestra revisión, se incluyeron estudios en inglés de bases de datos comunes como Google Scholar, Pubmed/MEDLINE, Scopus, Web of Science y la Biblioteca Cochrane con las palabras clave “prepartum”, “postpartum” y “peripartum”, combinadas con palabras clave que implican “accidente cerebrovascular materno” y “detección”. La fecha de finalización de esta revisión es septiembre de 2023.
Novedad científica: existen varios estudios que intentaron determinar la incidencia, los factores de riesgo, el tratamiento y las complicaciones del ictus materno; sin embargo, no existen estudios que intentaran identificar una herramienta de predicción precisa para el ictus periparto. En nuestro artículo, intentamos identificar una herramienta de predicción precisa para el ictus periparto.
Conclusiones: nuestra comprensión del ictus materno todavía tiene varias limitaciones. Aunque se han establecido indicadores de riesgo, actualmente no se dispone de técnicas para determinar qué mujeres tienen más probabilidades de sufrir ictus posparto y necesitan una observación más estrecha. El riesgo de un ictus materno posterior no se ha evaluado claramente, lo que limita los consejos que los clínicos pueden dar a las pacientes.
Palabras clave: Síndrome de Vasoconstricción Cerebral Reversible; Accidente Cerebrovascular Materno; Trombosis Venosa Cerebral; Hemorragia Intracraneal; Preeclampsia.
INTRODUCTION
Every 100,000 pregnancies, a maternal stroke complicates 30 of them and is accompanied by significant maternal mortality and morbidity. Hypertensive conditions of pregnancy (HDP), such as preeclampsia, gestational hypertension (HTN), migraine headaches, chronic HTN, eclampsia, antiphospholipid syndrome, and other thrombophilias, sickle cell disease, being a person of color, cesarean delivery, and extreme ages are all risk factors for peripartum stroke.(1)
Pregnancy-related stroke rates have been rising, paralleling the rise in cardiac conditions, obesity, and HTN among women of reproductive age. Race, smoking, age greater than 20 or less than 35, obesity, HTN, autoimmune disorders, sickle cell disease, patent foramen ovale, migraine headaches with aura, and vascular abnormalities are risk factors for pregnancy-related stroke. Smokers make up about two-thirds of the women who have a stroke related to pregnancy. In women of childbearing age, an elevated body mass index (BMI) is linked to a higher risk of ischemic stroke. The incidence and severity of strokes are related to race and ethnicity.(2)
In the US, stroke is the leading cause of serious maternal mortality and morbidity, killing 1 in 12 postpartum and pregnant females. For mothers who survive strokes, even nonfatal strokes, including those deemed “minor,” can cause severe disability and have long-term emotional, physical, and financial impact. Maternal stroke risk is influenced by the distinct pathophysiology and physiology of pregnancy, which can also lead to atypical stroke causes and presentations. The mechanisms, risk factors, and causes of maternal stroke must be understood by neurologists in order to properly diagnose, treat, and prevent this potentially fatal group of illnesses. The neurologist can be extremely important in both primary and secondary prevention as well as in the acute identification and management of maternal stroke. The pathophysiology, epidemiology, and management of maternal stroke are succinctly discussed in this article, with a focus on recent advances in the field.(3) The objective of the review is to analyze and identify the risk factors, incidence rates, predictor tools, and impacts of peripartum stroke on both the fetus and the mother.
Research focus
In our review, we are focusing on identifying peripartum stroke risk factors, incidence rates, predictor tools, and their impacts on both the fetus and the mother.
Research problem
Although it’s uncommon, ischemic stroke (IS) during puerperium and pregnancy can be a significant and distressing event for females, babies, and families. When it does happen, several worries about the fetus’s and the mother’s safety in connection to usual diagnostic procedures and treatments give rise to a more cautious approach.
Research questions
1. What are the causes of peripartum stroke?
2. What are the complications of peripartum stroke?
3. What are the risk factors of peripartum stroke?
4. How can we predict the occurrence of peripartum stroke?
5. What is the management of peripartum stroke?
Research aim
Our study aims to comprehensively investigate and demonstrate the prediction tools, risk factors, and predisposing conditions associated with peripartum stroke. Additionally, we seek to analyze the impact of peripartum stroke on both the fetus and the mother, providing detailed insights into these critical aspects of maternal health.
METHOD
General background
The American Heart Association/American Stroke Association (AHA/ASA) emphasized the necessity for focused studies to identify and characterize stroke risk factors specific to females with the release of its first-ever guidelines for stroke prevention in females, demonstrating pregnancy, especially as an area with inadequate data to make recommendations regarding prevention or screening. Stroke is an uncommon peripartum event that has a modest incidence (34,2 per 100,000 deliveries) but potentially serious consequences. According to prior articles, postpartum females are at the greatest risk of both hemorrhagic and ischemic stroke, with a 3-fold greater risk of stroke. It is alarming that the prevalence of pregnancy-associated stroke (PAS) seems to be rising.
Preeclampsia/eclampsia, chronic kidney disease (CKD), cesarean section, HTN, pregnancy-related hematologic diseases, black race, migraine, older age, primary hypercoagulable states, gestational diabetes, and smoking are risk factors for PAS that have been reported in prior studies. However, scientists still don’t fully comprehend the intricate mechanisms that underlie PAS. The majority of research has included both ischemic and hemorrhagic strokes without further describing each category. Furthermore, prior research relied on sizable administrative datasets without doing a comprehensive evaluation of the ischemic stroke subtype and uncommon related diseases such as cervical artery dissections, RCVS, and CVT.
Inclusion criteria
1. The design of the articles is a randomized clinical trial, case series, case-control, or systematic review.
2. The aim of the study should be the detection of peripartum stroke.
3. Most of the included studies should be recent, from 2018 to 2023.
4. Studies that related to the risk factors of peripartum stroke.
Exclusion criteria
1. Studies and articles that were not peer-reviewed, as well as proposals, procedures, letters, and opinions.
2. Old studies that were conducted before 2010.
3. Studies not related to our topic or their aim were not related to ours.
Information sources
A review of English studies was conducted using common databases such as Pubmed/MEDLINE, Google Scholar, Web of Science, Scopus, and the Cochrane Library.
The search was conducted using the keywords “prepartum,” “postpartum,” and “peripartum,” combined with keywords involving “maternal stroke,’’ and ‘’detection.” The end date for this review is September 2023. We collected studies using each set of keyword combinations to create an unbiased collection of publications. We excluded studies and articles that were not peer-reviewed, as well as proposals, procedures, letters, and opinions. The references included in this paper were chosen because they are relevant to our topic. The focus of this paper is to demonstrate the prediction tools for risk factors and predisposing factors of peripartum stroke and its impact on the fetus and the mother.
Data collection
The included articles were reviewed in three stages. The first stage involved utilizing EndNote Software to import the findings from electronic databases into a Microsoft Excel sheet. The articles entered into the Excel sheet were screened for titles and abstracts in the second stage. The third stage involved screening the included citations from Stage 2’s full text. In addition, we manually checked the included publications’ references for any potentially overlooked studies.
Statistical analysis
A qualitative analysis was conducted on previously published papers to reach the study’s conclusions. Given the nature of the study as a narrative review, a quantitative analysis was not feasible. The quantitive analysis needs to specify the outcomes that you will measure and to find more than two studies that reported the data of these outcomes, then compare these data to get the conclusion. We tried to do a quantitive analysis in our study, but we could not specify outcomes related to our topic or studies that reported the data of the same outcome. So, we did a qualitative analysis using studies related to our topic, reported their results and conclusions, and compared them with each other to reach good evidence and recent results and conclusions.
Literature review
Stroke risk is higher following delivery than it was during pregnancy, according to numerous research. However, 30–40 % of strokes related to pregnancy happen during delivery admission, and according to at least one study, the risk is highest one day before and two days following birth. Despite known risk factors, these catastrophic occurrences are rare and, hence, impossible to forecast, which makes it challenging to develop preventive measures.(4)
Infection is a new risk factor for stroke that has become a significant and underestimated cause, especially in young individuals. In a large population-based investigation, urinary tract infections and acute respiratory infections were linked to a brief rise in the incidence of cardiovascular events like myocardial infarction and stroke.(5) The odds of postpartum stroke were 25 times higher in females with postpartum infections, according to one study, which revealed a link between infections and postpartum or peripartum stroke.(6) Pregnant females with preeclampsia, a subgroup that has a 5- to 6-fold higher risk of pregnancy-related stroke than pregnant females without preeclampsia, have infections as a risk factor for peripartum stroke.(7) A number of studies have shown a substantial correlation between preexisting infections such as Helicobacter pylori, urinary tract infections, and Chlamydia pneumonia and the onset of preeclampsia.(8) This is intriguing because preeclampsia’s pathogenesis has also been linked to infections. The relationship between infection and peripartum stroke in the general pregnant population is unclear.(9) There is a dearth of information on the timing of infections (existing at admission or hospital-acquired), and previous studies have not shown infections as a factor in birth-related strokes. Furthermore, the majority of earlier investigations of pregnancy-related stroke did not consider infection subtypes as a risk factor.(10)
In order to support health system planning and clinical decision-making, it is critical to have precise estimates of the rate of stroke during and immediately after pregnancy.(11) This is because stroke has a significant impact on females of childbearing age, their health systems, and families, and because both obstetrical care and stroke are becoming increasingly organized internationally.(12,13) This study’s objectives were to conduct a review to find studies on the incidence of stroke in pregnancy and investigate the effects of geography, time, and methodology.
Due to the uncommon nature of the events, the absence of prospective research, and the absence of postpartum or pregnant females from clinical stroke trials, there are few clinical data available to guide preventive measures and therapy for maternal stroke.(14) However, observational research and translational work, such as employing preeclampsia animal models, can shed light on the specific components that contribute to the pathophysiology of maternal ischemia and HS and may help to guide acute care.(15)
Although uncommon, hemorrhagic and ischemic strokes are extremely morbid problems that can occur during the puerperium and pregnancy. According to a recent estimate, all subtypes together, there are about 30 maternal strokes per 100,000 pregnancies.(16) Maternal HS and IS combined incidence in high-risk populations, such as preeclampsia in pregnant females and other hypertensive diseases of pregnancy, is up to six times higher than in pregnant females without these conditions. In addition to increasing the risk of miscarriage, pregnancy-related stroke can impair a woman’s ability to care for her children, pursue her career, and take care of herself. The epidemiology, pathophysiology, risk factors, and treatment of maternal stroke are reviewed in this focused update, and the role of the obstetric anesthesiologist in the detection and peripartum care of this potentially disastrous occurrence is also covered.(17)
Stroke definitions and subtypes
The American Heart Association/American Stroke Association’s (AHA/ASA) comprehensive definition of “stroke” includes IS caused by an arterial or venous infarction of the central nervous system as well as HS, which includes nontraumatic subarachnoid hemorrhage (SAH) and intracerebral hemorrhage (ICH).(18) Due to venous congestion, cerebral venous thrombosis (CVT) can cause ICH or SAH. It might be difficult to understand the variety of stroke diagnoses, and the acute therapy varies based on the precise stroke etiology. Unless otherwise stated, the term «stroke» shall refer to the comprehensive AHA/ASA definition (IS and HS); nonetheless, each stroke subtype›s etiology and treatment will be covered separately.(19)
Epidemiology
When compared to non-pregnant females of the same age, the risk of stroke is almost three times higher in postpartum and pregnant females. The rate of maternal stroke was 30 per 100,000 pregnancies in a meta-analysis of 11 articles involving more than 85 million postpartum and pregnant females from high-income countries; the majority occurred in the postpartum duration (up to 6 weeks)(20). Small studies from several nations show an alarmingly greater prevalence of maternal stroke in the middle- and low-income countries, regardless of a lack of reliable large-scale studies in this area. Maternal stroke prevalence was 89 per 100,000 births in one study conducted in Tanzania between 2009 and 2010 involving more than 5,500 deliveries.(21) Another study of more than 39,000 births in India between 2006 and 2008 found that there were 66 cerebrovascular problems for every 100,000 births.(22)
Timing of maternal stroke
Most maternal strokes happen during postpartum times, which can range from one to twelve weeks following birth. This is frequently after the females are out of the hospital. The median duration to readmission for stroke following delivery was 8 days, according to a study utilizing administrative information from the US Healthcare Cost and Utilisation Project’s Nationwide Readmissions Database during 2013–2014.(23) According to estimates, the risk of thromboembolic complications during the first week after giving birth is 15–35 times higher in postpartum females than in non-pregnant females, and the risk persists through week 12. A case-crossover study employing administrative information from New York, California, and Florida discovered a 9-fold greater rate of HS in the 12 weeks post-partum compared to the non-pregnant condition.(24)
Changes in the mother during pregnancy that increase the risk of ischemic stroke
As a result of changes in hormonal status during pregnancy, the hemostatic and hemodynamic systems undergo changes that affect the maternal physiological state. It is currently unknown if this adaptation would impact ischemic stroke risk, and the relationship between the two is probably complicated. The overall balance swings towards a hypercoagulant effect during pregnancy, which is typically accompanied by considerable alterations in venous flow and the molecular mediators of hemostasis (table 1).(19)
Procoagulant alterations become more pronounced around the term and in the first few days after delivery, which is likely due to the placenta’s evacuation and the production of thermoplastic chemicals at the site of separation. Three weeks after delivery, fibrinolysis and blood coagulation return to their pre-pregnancy levels. In particular, during the third trimester and the puerperium, this ensuing hypercoagulable state, in combination with a venous stasis condition, may likely be responsible for an elevated risk of thromboembolic consequences. An increase in total body water during the first 10 weeks of pregnancy causes a volume shift, which persists until 1 to 2 weeks following birth before gradually returning to normal.(25,26)
|
Table 1. Modifications of haemostatic factors during pregnancy(19) |
|
|
Procoagulant factors |
|
|
Factor XI |
C |
|
von Willebrand factor |
↑ |
|
Factors VII, VIII, IX, X, XII |
↑ |
|
Factor II |
C |
|
Fibrinogen (factor I) |
↑ |
|
Factors V, XIII |
↑↓ |
|
Coagulation inhibitors |
|
|
Protein C, antithrombin III |
= |
|
Protein S |
↓ |
|
Fibrinolytic factors |
|
|
Thrombin activatable fibrinolysis inhibitor (TAFI) |
↑ |
|
Plasminogen activator inhibitor 1 and 2 (PAI-1, PAI-2) |
↑ |
|
Tissue plasminogen activator |
↓ |
|
Others |
|
|
D-dimer, fibrinopeptide-A |
↑ |
|
Thrombin-antithrombin complex |
↑ |
|
Prothrombin fragment 1+2 |
↑ |
|
Platelet count |
↓ |
|
↓: decrease; ↑: increase; ↑↓: early increase followed by decrease; =: no significant change; C: controversial data. |
|
Heart rate, cardiac output, and stroke volume all increase by 30 % to 50 % as a result of this hypervolemic condition, in addition to the growing circulatory needs of the fetus and placenta. The first eight weeks of pregnancy account for half of this shift, which peaks between 25 and 30 weeks. The cardiac output changes dramatically throughout labor and rises steadily as labor progresses, increasing by a mean value of 30 %.(27) Additionally, the heart rate constantly rose. Then, in the first few days following birth, there is a sharp decline in stroke volume and heart rate. Gradually, by two weeks of delivery, cardiac output falls to 50 % above the prepregnancy level, and between six and twelve weeks later, it recovers to normal. Because of the reduction in systemic vascular resistance, blood pressure begins to decline during the seventh week, reaches its lowest point between 24 and 32 weeks, and gradually rises to pre-pregnancy values at term.(28) During pregnancy, venous compliance rises, which causes a reduction in blood flow, an increase in stasis, and a propensity for orthostatic pressure dips. During pregnancy, it has also been noted that the collagen and elastin contents of the artery wall change, and there is a loss of distensibility that partially returns to normal at term.
Common mechanisms of stroke during postpartum and pregnancy
Similar to young adults who are not pregnant, postpartum and pregnancy stroke mechanisms are frequently uncommon. These typical stroke-related mechanisms are outlined below.
Arterial Ischemic Stroke
Cardioembolism, which is brought on by paradoxical embolism in the presence of pulmonary shunt or patent foramen ovale, previous cardiac illness, or cardiomyopathy during pregnancy, is the most frequent cause of arterial IS during postpartum and pregnancy. Cervical artery dissections, arterial thrombosis brought on by thrombophilias, arterial vasospasm in conjunction with RCVS, or more uncommon conditions like moyamoya vasculopathy are some additional causes of arterial ischemic stroke.(29,30)
Cervical Artery Dissection
Cervical artery dissections related to pregnancy have been described frequently with postpartum HTN, RCVS, or both. Pregnancy raised the chance of cervical artery dissection more than fivefold, according to case-control research utilizing data from New York and Florida; this elevated risk was only evident in the postpartum duration and not in the antepartum duration. Nearly half of the females who underwent dissections had hypertensive problems during pregnancy, and all of the incidents took place following hospital discharge for birth. Cervical artery dissection frequently results from trauma, and doctors should be aware that intimate partner abuse is widespread throughout pregnancy and the postpartum duration.(2,31)
Cerebral Venous Thrombosis
Timing and risk factors. When a clot forms in the cerebral cortical veins or the dural sinuses, CVT results. Although CVT may not necessarily end in stroke, the blood-brain barrier’s collapse and venous congestion might cause venous infarction and/or hemorrhage. The puerperium is one of the more frequent causes of CVT, which is probably caused by endothelial injury, venous stasis, and hypercoagulability. There have also been reports of CVT linked to accidental dural puncture during epidural catheter implantation. The incidence of puerperal CVT is increased by HTN, infections, and cesarean delivery.(32,33) Increased intracranial pressure (ICP), cerebral edoema, venous congestion, infarction, and hemorrhage may occur as the venous clot spreads. Contrary to the typical “thunderclap” headache of subarachnoid hemorrhage, the development of CVT symptoms is frequently sneaky. Only when a headache becomes excruciating, when neurologic signs appear, or when catastrophic bleeding takes place may a patient seek medical assistance. Inflammation and infections with bacteria, fungi, viruses, and some parasites are becoming implicated as CVT and IS risk factors and “triggers” in susceptible people, including pregnant females.(34,35)
Intracerebral Hemorrhage
The most frequent occurrence of ICH in pregnancy is in the presence of hypertensive disease of pregnancy. According to autopsy studies, ICH is the primary factor in about one-third of preeclampsia-related deaths. ICH is frequently observed in conjunction with posterior reversible encephalopathy syndrome (PRES), which can occur both during and outside of pregnancy and is characterized by endothelial dysfunction, posterior-predominant vasogenic edema of the brain, and HTN.(36) Nearly all patients with eclampsia have PRES symptoms on MRI, and PRES is strongly related to eclampsia in pregnant or postpartum patients(37). Although PRES can be reversed, it can potentially lead to life-threatening side effects such as cerebral edoema and ICH; thus, it needs to be properly watched. Additionally, preexisting vascular lesions such as cerebral cavernous malformations, brain arteriovenous malformations, brain aneurysms, or unstable moyamoya collaterals may rupture and result in ICH (figure 1).(38)
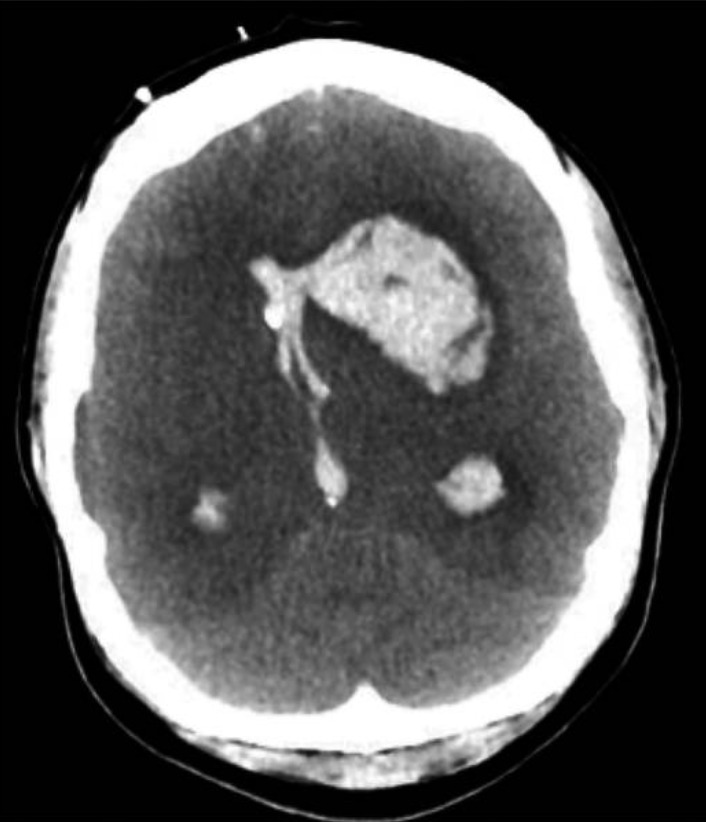
Figure 1. Imaging of the patient with eclampsia and fatal ICH (38)
Reversible Cerebral Vasoconstriction Syndrome
The RCVS syndrome is characterized by segmental, multifocal, transient, nonvasculitic vasospasm of the medium and large-sized cerebral vessels, and it frequently manifests as recurrent thunderclap headaches without or with corresponding neurologic impairments.(39) The majority of times, RCVS is brought on by an orgasmic experience, a serotonergic or sympathomimetic substance (such as cocaine or antidepressants), or the postpartum period, which can account for up to 20 % of occurrences. Similar to PRES, although RCVS vasculopathy is inherently reversible, the sequelae may not be severe, and fulminant RCVS may cause ischemic stroke, ICH, and/or SAH, which can have life-altering effects.(40) Patients with preeclampsia are more likely to experience PRES and RCVS, and these two conditions frequently coexist. This could be because endothelial failure, inflammation, and sympathetic hyperactivity, which are all associated with preeclampsia, share a common pathogenesis (figure 2).(38)
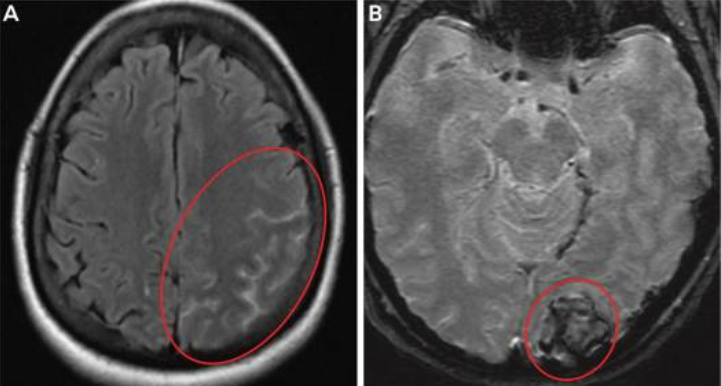
Figure 2. Imaging of the patient with preeclampsia and RCVS.
A, MRI shows a diffuse left-sided convexity subarachnoid hemorrhage (circled).
B, shows a left occipital intracerebral hemorrhage (circled).(38)
Risk factors
Patient Characteristics
According to a study of the Nationwide Inpatient Sample, the absolute risk of stroke increased with age: patients over 40 had an odds ratio (OR) for stroke of 2,0 (95 % confidence interval (CI) 1,4-2,7, P 0,01), compared to patients under 20 who had an OR of 2,0 (95 % CI 1,4-2,7, P 0,01).(41) This was validated in a subsequent investigation using the Nationwide Inpatient Sample, which revealed a significantly greater risk of acute stroke among females under the age of 45 during puerperium and pregnancy. Interestingly, a study indicated that the risk of stroke increased during postpartum and the pregnancy duration for younger females but not for older females. However, pregnancy at an older age may have detrimental effects on the health of the cerebrovascular system in later life.(42) Females from the observational cohort of the Females’s Health Study who were 59 to 70 years old had their stroke risk according to the age of their most recent pregnancy or birth examined. In multivariate analysis, females who were older at delivery (age 40 years) had a somewhat higher risk of Hs compared to females who were younger at delivery (OR 1,5, 95 % CI 1,0-2,1). Females over the age of 40 had atrial fibrillation, higher mean SBP, heart failure, higher rates of diabetes mellitus, and any alcohol use later in life compared to younger females at the time of delivery.(43)
Medical Risk Factors
The risk of stroke is increased by the presence of vascular risk factors during pregnancy. Stroke risk factors include thrombophilia, migraine, heart disease, systemic lupus erythematosus, HTN, sickle cell disease, thrombocytopenia, and diabetes. Pregnancy-related migraine-related stroke may be mediated by HDP. Pregnancy-related stroke is also strongly predicted by pregnancy-related complications such as postpartum infections, transfusion, and any form of infection at the time of delivery hospitalization, particularly genitourinary infections, and sepsis. At a median of 6,7 days, it was discovered that infection at delivery admission was a risk factor for stroke readmission.(44)
In contrast to hemorrhagic stroke, this connection was substantial for readmissions brought on by postpartum ischemic stroke. The length of the hospital stay during birth, which may be a sign of higher morbidity, is a separate risk factor for maternal stroke. Additionally, smoking is a significant risk factor for maternal stroke and is particularly prevalent in females who experience strokes during pregnancy.(45) Other pregnancy-specific causes are uncommon and include choriocarcinoma, peripartum cardiomyopathy, and embolization of amniotic fluid or air. Due to the greater incidence of pregnancy-related venous thrombosis, it is hypothesized that ischemic strokes owing to transcardiac embolism via a patent foramen ovale may happen during pregnancy. A review article, however, only identified a limited number of these instances, the majority of which happened between the first and second pregnancy trimesters.(46)
Management of peripartum stroke
Delivery of the infant and the afflicted placenta is the only proven remedy for preeclampsia. Pregnant females who develop preeclampsia before 37 weeks should be delivered. Unless symptoms of a severe disease appear, expectant management should be used for females whose preeclampsia starts before 37 weeks of gestation (ideally with maternal-fetal medicine involvement for 34 weeks of gestational age).(7)
The risk of preeclampsia in females was first shown to be decreased by aspirin in 1979. After that, it was investigated in numerous clinical studies. According to a recent study, aspirin 150 mg daily from weeks 11–14 to week 36 of gestation was more effective than a placebo in preventing pre-term preeclampsia (1,6 % vs. 4,3 %, respectively; OR 0,38, 95 % CI 0,20–0,74) in patients who were at a high risk of developing the condition. This was achieved without raising the risk of adverse neonatal outcomes. In the International Society for the Study of HTN in Pregnancy’s (ISSHP) newly released consensus statement on hypertensive diseases of pregnancy, this medication is advised.(47)
Pre-eclampsia has been linked to a lower incidence of high calcium consumption during pregnancy. Calcium may exert this effect by reducing the production of parathyroid hormone and intracellular calcium, which in turn causes a reduction in the contractility of smooth muscle. Additionally, calcium supplementation may lessen the contractility of the uterine smooth muscle and stop preterm labor and delivery. In addition to aspirin, calcium supplementation of 1,2–2,5 g/day is advised for preeclampsia-prone females with inadequate calcium intake or whose daily calcium requirements are unknown.(48)
Females with severe HTN (BP 160/110) during pregnancy or puerperium should have their blood pressure monitored and managed with medications such as oral nifedipine or intravenous labetalol. Different medical societies have different suggestions for treating mild HTN. For instance, the treatment of mild to moderate HTN is not advised by the American College of Obstetricians and Gynaecologists.(48)
For preeclamptic females who have severe HTN, proteinuria, or HTN with neurological symptoms or indications, this is advised. It has been demonstrated that the administration of magnesium sulfate, when compared to placebo, reduces the rate of development of eclampsia in preeclamptic females with severe characteristics by 58 %. In addition, magnesium sulfate is superior to phenytoin and diazepam for preventing repeated seizures in eclampsia.(48)
RESULTS
A search was conducted using a specified search strategy, resulting in the identification of 6,660 articles. These articles were then screened to select those relevant to the topic of study. After excluding articles based on title and abstract screening, we conducted a full-text screening of 280 articles. Ultimately, we used 58 articles to gather information about our topic and write this review (figure 3).
Figure 3. Results of our search
Recent increases in the prevalence of hypertensive conditions during pregnancy may be to blame for the rise in the frequency of maternal stroke. The early postpartum and peripartum durations are when there is the greatest risk of a maternal stroke. Preeclampsia is strongly linked to RCVS, posterior reversible encephalopathy syndrome, and a higher risk of stroke and vascular dementia over the long term. Maternal stroke risk factors involve hypertensive conditions of pregnancy, migraines, and infections. Few data are known about the safety of thrombolytics in the postpartum period, although limited evidence suggests that endovascular reperfusion and thrombolytic therapy are safe and efficacious in pregnant females who have had IS. There are now updated consensus guidelines to help in the treatment of HS and IS during pregnancy.
DISCUSSION
According to several earlier research, male patients had a larger percentage of strokes than female patients did. Increased male risk factors, including drinking alcohol and smoking cigarettes, could be the cause. Additionally, endogenous estrogens in males are not vascularly protected.(49,50) This was in contrast to several studies where female patients predominated; this may be because females were more likely to use contraception, experience illnesses related to pregnancy, and suffer strokes from migraines in those studies. The bulk of the patients in our study were people who lived in rural areas.(49,50) The bulk of the patients were, however, from metropolitan regions, according to research by Gebremariam (2016)(51) and Greffie (2015).(52) It is obvious that the kind of people that visit hospitals vary across hospital-based cohorts. The type of patients who attend the hospital depends on its location and catchment area. Additionally, the age composition of populations in urban and rural areas may vary. The increased prevalence of stroke in rural areas may potentially be a result of low-risk factor awareness and inadequate risk factor management.(53)
In 75,9 % of cases, HTN was shown to be the primary risk factor, which is consistent with earlier research, as uncontrolled HTN is the primary cause of stroke in both developed and developing nations. This pattern may be a result of a lack of healthcare access, poor health practices, and community awareness of the issue. Even when offered therapy for HTN, black people are less likely than white people to stick to their prescribed course of action. Due to a lack of an active screening program, the failure to conduct routine blood pressure readings, poor medical history taking, and poor patient follow-up, we feel that HTN is underdiagnosed and undertreated in our study community. Additionally, since uncomplicated HTN is typically asymptomatic and denial of the disease is frequent, maintaining adherence to long-term treatment is extremely difficult to attain the best results.(34,35)
A global rate of 24 per 100,000 person-years for all peripartum-related strokes (PRS) was observed, spanning the peripartum, antepartum, and up to six weeks post-delivery. This included 42,9 % ischemic strokes (IS), 41,9 % hemorrhagic strokes (HS), and 17,4 % cerebral venous thrombosis (CVT).
Particularly in HS, the stroke incidence occurring during the peripartum was significantly higher than during other times. Pregnant females had a CVT risk that was more than eight times greater than that of non-pregnant females. From 2010 to 2018, we also observed an increase in the rate of all types of strokes in pregnant females, with the temporal increase in the rate of IS mostly accounting for this trend. Pregnancy-related HTN problems were identified in more than 40 % of pregnancies who suffered from HS. Regional differences were seen, with the French overseas territories having the highest rates of stroke.
The postpartum and peripartum periods had a heightened risk of stroke.(50) In order to explain this finding, it is possible to hypothesize that the Valsalva maneuver used by the mother during labor could cause an increase in intracranial blood pressure, which could result in a stroke, particularly HS. Pre-eclampsia and HELLP syndrome,(54) two hypertensive diseases of pregnancy, were put forth in the aforementioned Finnish study as possible explanations for the increased rate of all stroke types during the peripartum period. Prior research found that compared to non-pregnant females, the risk of thromboembolic events was measured to be 15 to 35 times higher during the first postpartum week.(55) It was proposed that the greater risk of stroke after postpartum may be related to a significant drop in blood volume or swift hormonal changes following childbirth, possibly through changes in hemodynamics, coagulation, or vessel walls.(56)
Our research found that the rate of stroke has been rising over time. Even after accounting for the older age of the cohort of females between 2010 and 2018, this tendency persisted. According to an American study, there was a 61,5 % temporal increase between 1995 and 2010–2011.(57) The observed increase was mostly brought on by the rise in IS incidence, as previously documented by Karjalainen et al.(54) (2021). This temporal increase may be attributable to improved diagnostic methods, particularly for IS, which manifests mild symptoms, or to an increase in the prevalence of comorbid illnesses in pregnant females, such as HTN and cardiac disease.(50) In France, where there has been an increase in the incidence of IS, a comparable temporal trend has been found in the young general adult population.(53) In contrast to other strokes, hypertensive conditions of pregnancy were frequently seen in HS, as shown in other research, but traditional cardiovascular risk factors (obesity, diabetes, cigarette use, and chronic HTN) were regularly identified in IS.(58) Early detection and monitoring of HTN conditions during pregnancy are required to lower the risk of cardiovascular complications due to the high prevalence of these conditions in pregnancy-related strokes. Low socioeconomic level, gestational diabetes, obesity, and a personal history of VTE were significant risk factors for CVT in females. Karjalainen et al.(54) (2021) discovered that the females with CVT frequently had the traits of obesity and gestational diabetes; however, unlike us, they also identified diabetes as a key characteristic. The article by Sukhostavets (2022) focuses on the psycho-rehabilitation and adaptation of pregnant women and mothers in the postpartum period who have experienced traumatic events during the war. The study highlights the psychological and physical impacts of such trauma on this group. The findings and insights from this research could be significant in identifying pregnancy-related risk factors, including stress and mental health issues, which might contribute to peripartum stroke. Understanding these factors is crucial for developing effective prediction and early detection tools for peripartum stroke, thereby improving maternal and fetal health outcomes.(59) The findings underline the necessity of holistic healthcare that considers mental well-being.
This study is significant as it provides comprehensive insights into the factors contributing to peripartum stroke, emphasizing the role of socio-economic factors, healthcare access, and regional disparities. Its findings are crucial for developing targeted interventions and preventive measures to reduce the incidence of peripartum stroke, ultimately improving maternal and fetal health outcomes. The study’s emphasis on the rising trend of stroke and its associated risk factors highlights the urgent need for enhanced public health strategies and personalized healthcare approaches.
Limitations
This article’s primary drawback is that it is a narrative review. A narrative review describes the findings of the included research in written paragraphs. They don’t use the information from the summarised studies to perform any pooled analysis. This precludes pooled analysis and, hence, real objectivity. Rather, a narrative review serves as a compiled resource of the prevalent viewpoints at the time of publication. This can serve as a suitable method for gaining a thorough comprehension of a body of evidence. As it does not thoroughly consider the alternative hypothesis, it does not guarantee that the prevailing ideas are true.
CONCLUSION
It is becoming more well-acknowledged that maternal stroke is a significant factor in the rising rates of maternal mortality and morbidity. Our knowledge of the elements that might cause a maternal stroke, however, still has a lot of holes. For example, although several risk factors for maternal stroke have been discovered, no prediction tools exist to assist in determining which females may be most at risk for peripartum stroke and need closer monitoring. Stroke risk factors include thrombophilia, migraine, heart disease, systemic lupus erythematosus, HTN, sickle cell disease, thrombocytopenia, and diabetes. Pregnancy-related migraine-related stroke may be mediated by HDP. Pregnancy-related stroke is also strongly predicted by pregnancy-related complications such as postpartum infections, transfusion, and any form of infection at the time of delivery hospitalization, particularly genitourinary infections, and sepsis. Clinicians find it challenging to provide patients advice because the risk of recurrent maternal stroke, particularly hemorrhagic stroke, has not been sufficiently quantified. Preeclampsia, other HDPs, and their effects on the cerebral vasculature require more in-depth research using animal models because of their complicated pathophysiology. Similar to this, it is unclear what, exactly, causes migraine and maternal stroke. The function of the maternal immune system and the processes by which infection may raise the risk of maternal stroke are still poorly understood. Maternal stroke genomic and genetic risk factors have not been studied. Fortunately, the research and clinical communities are becoming increasingly interested in figuring out the pathogenesis of maternal stroke. Over the past 20 years, the number of articles examining the relationship between stroke and pregnancy has rapidly increased. National attention has been drawn to the problem of maternal mortality and morbidity, which has led to the creation of publicly financed initiatives like New York State’s Safe Motherhood Initiative. The moment is now to place more emphasis on maternal stroke prevention, detection, and treatment as a crucial component of enhancing maternal outcomes. It is possible to hypothesize that the Valsalva maneuver used by the mother during labor could cause an increase in intracranial blood pressure, which could result in a stroke, particularly HS. Our findings will be good material for conducting new studies that try to find accurate tools for prediction of peripartum stroke. However, if they can not find any tools for prediction and early diagnosis of peripartum stroke, the objectives of the new studies should change to determine the risk factors and evaluate the preventive measures that may help in decreasing the incidence of maternal strokes.
REFERENCES
1. Miller EC. Maternal Stroke Associated With Pregnancy. Contin Lifelong Learn Neurol [Internet]. 2022 Feb;28(1):93–121. Available from: https://journals.lww.com/10.1212/CON.0000000000001078
2. Salehi Omran S, Parikh NS, Poisson S, Armstrong J, Merkler AE, Prabhu M, et al. Association between Pregnancy and Cervical Artery Dissection. Ann Neurol [Internet]. 2020 Sep 13;88(3):596–602. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ana.25813
3. Zambrano MD, Miller EC. Maternal Stroke: an Update. Curr Atheroscler Rep [Internet]. 2019 Sep 22;21(9):33. Available from: http://link.springer.com/10.1007/s11883-019-0798-2
4. Kusainov A. Optimising anesthesia support during operations on the abdominal aorta and its branches. Futur Med [Internet]. 2022 Sep 30;11–21. Available from: http://futurity-medicine.com/index.php/fm/article/view/12
5. Elgendy IY, Bukhari S, Barakat AF, Pepine CJ, Lindley KJ, Miller EC. Maternal Stroke. Circulation [Internet]. 2021 Feb 16;143(7):727–38. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.051460
6. Baig S. Change in physical and mental health due to aging: future perspective. Futur Med [Internet]. 2023 Mar 30;13–23. Available from: http://futurity-medicine.com/index.php/fm/article/view/22
7. Kozberg MG, Camargo EC. Management of Maternal Stroke and Mitigating Risk. Curr Treat Options Cardiovasc Med [Internet]. 2019 Nov 21;21(11):72. Available from: http://link.springer.com/10.1007/s11936-019-0770-z
8. Khalid N. The role of artificial intelligence in the management of lung cancer: a narrative review. Futur Med [Internet]. 2023 Mar 30;36–46. Available from: http://futurity-medicine.com/index.php/fm/article/view/24
9. Maltsev D V., Hurzhii OO. Recurrent ocular toxoplasmosis infection in a patient with a selective deficiency of NK T-cells and cytotoxic СD8+ T-cells associated with a genetic folate cycle deficiency. Oftalmol Zh [Internet]. 2022 Aug 22;99(4):49–57. Available from: https://www.ozhurnal.com/en/archive/2022/4/8-fulltext
10. Brohan MP, Daly FP, Kelly L, McCarthy FP, Khashan AS, Kublickiene K, et al. Hypertensive disorders of pregnancy and long-term risk of maternal stroke—a systematic review and meta-analysis. Am J Obstet Gynecol [Internet]. 2023 Sep;229(3):248–68. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002937823001977
11. Diachenko I, Kalishchuk S, Zhylin M, Kyyko A, Volkova Y. Color education: A study on methods of influence on memory. Heliyon [Internet]. 2022 Nov;8(11):e11607. Available from: https://linkinghub.elsevier.com/retrieve/pii/S240584402202895X
12. Miller EC, Zambrano Espinoza MD, Huang Y, Friedman AM, Boehme AK, Bello NA, et al. Maternal Race/Ethnicity, Hypertension, and Risk for Stroke During Delivery Admission. J Am Heart Assoc [Internet]. 2020 Feb 4;9(3). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.119.014775
13. Roeder HJ, Lopez JR, Miller EC. Ischemic stroke and cerebral venous sinus thrombosis in pregnancy. In: Handbook of Clinical Neurology [Internet]. 2020. p. 3–31. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780444642400000015
14. Foo L, Bewley S, Rudd A. Maternal death from stroke: a thirty year national retrospective review. Eur J Obstet Gynecol Reprod Biol [Internet]. 2013 Dec;171(2):266–70. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0301211513004715
15. Miller EC, Leffert L. Maternal Stroke. In: Principles and Practice of Maternal Critical Care [Internet]. Cham: Springer International Publishing; 2020. p. 343–61. Available from: http://link.springer.com/10.1007/978-3-030-43477-9_25
16. Bandoli G, Baer RJ, Gano D, Pawlowski LJ, Chambers C. Migraines During Pregnancy and the Risk of Maternal Stroke. JAMA Neurol [Internet]. 2020 Sep 1;77(9):1177. Available from: https://jamanetwork.com/journals/jamaneurology/fullarticle/2766569
17. Gulati AK, Castri P, Deveber GA, Menon BK, Majersik JJ, Clark E, et al. Abstract WP183: Pregnancy-related Factors Associated With Stroke In Mothers And Newborns: Data From The International Maternal Newborn Stroke Registry. Stroke [Internet]. 2022 Feb;53(Suppl_1). Available from: https://www.ahajournals.org/doi/10.1161/str.53.suppl_1.WP183
18. Ylinen A, Hägg-Holmberg S, Eriksson MI, Forsblom C, Harjutsalo V, Putaala J, et al. The impact of parental risk factors on the risk of stroke in type 1 diabetes. Acta Diabetol [Internet]. 2021 Jul 15;58(7):911–7. Available from: https://link.springer.com/10.1007/s00592-021-01694-x
19. Rossignol M, Jonard M, Cohen H. Mortalité maternelle par accident vasculaire cérébral en France 2013–2015. Gynécologie Obs Fertil Sénologie [Internet]. 2021 Jan;49(1):73–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2468718920303354
20. Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert L, Bushnell C, et al. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. Int J Stroke [Internet]. 2017 Oct 8;12(7):687–97. Available from: http://journals.sagepub.com/doi/10.1177/1747493017723271
21. Ndaboine EM, Kihunrwa A, Rumanyika R, Im HB, Massinde AN. Maternal and perinatal outcomes among eclamptic patients admitted to Bugando Medical Centre, Mwanza, Tanzania. Afr J Reprod Health [Internet]. 2012 Mar;16(1):35–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22783666
22. Prabhu TRB. Cerebrovascular Complications in Pregnancy and Puerperium. J Obstet Gynecol India [Internet]. 2013 Apr 27;63(2):108–11. Available from: http://link.springer.com/10.1007/s13224-012-0251-8
23. Too G, Wen T, Boehme AK, Miller EC, Leffert LR, Attenello FJ, et al. Timing and Risk Factors of Postpartum Stroke. Obstet Gynecol [Internet]. 2018 Jan;131(1):70–8. Available from: https://journals.lww.com/00006250-201801000-00010
24. Meeks JR, Bambhroliya AB, Alex KM, Sheth SA, Savitz SI, Miller EC, et al. Association of Primary Intracerebral Hemorrhage With Pregnancy and the Postpartum Period. JAMA Netw Open [Internet]. 2020 Apr 14;3(4):e202769. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2764376
25. Malek AM, Wilson DA, Turan TN, Mateus J, Lackland DT, Hunt KJ. Maternal Coronary Heart Disease, Stroke, and Mortality Within 1, 3, and 5 Years of Delivery Among Women With Hypertensive Disorders of Pregnancy and Pre‐Pregnancy Hypertension. J Am Heart Assoc [Internet]. 2021 Mar 2;10(5). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.120.018155
26. Katsuragi S, Tanaka H, Hasegawa J, Nakamura M, Kanayama N, Nakata M, et al. Analysis of preventability of stroke-related maternal death from the nationwide registration system of maternal deaths in Japan. J Matern Neonatal Med [Internet]. 2018 Aug 18;31(16):2097–104. Available from: https://www.tandfonline.com/doi/full/10.1080/14767058.2017.1336222
27. Tanaka H, Hasegawa J, Katsuragi S, Tanaka K, Arakaki T, Nakamura M, et al. Are There Maternal Deaths Related to Hemorrhagic Stroke Due to Hypertensive Disorder of Pregnancy That Could Be Potentially Preventable by Tight Hypertension Management in Antepartum? A Retrospective Study from the Maternal Death Exploratory Committee in . J Clin Med [Internet]. 2023 Apr 17;12(8):2908. Available from: https://www.mdpi.com/2077-0383/12/8/2908
28. Waites BT, Walker AR, Caughey AB. Delivery timing in dichorionic diamniotic twin pregnancies complicated by preeclampsia: a decision analysis. J Matern Neonatal Med [Internet]. 2022 Dec 12;35(25):9780–5. Available from: https://www.tandfonline.com/doi/full/10.1080/14767058.2022.2053103
29. Morello A, Casseri T, Acampa M, Galluzzi P, Cerase A, Monti L. Stroke in Pregnancy and Review of Current Literature: Arterial Spin-Labeling MRI Can Identify the Presence and Intensity of Collateral Circle. J Stroke Cerebrovasc Dis [Internet]. 2018 Dec;27(12):3575–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1052305718304695
30. Geraldo AF, Parodi A, Bertamino M, Buffelli F, Uccella S, Tortora D, et al. Perinatal Arterial Ischemic Stroke in Fetal Vascular Malperfusion: A Case Series and Literature Review. Am J Neuroradiol [Internet]. 2020 Dec;41(12):2377–83. Available from: http://www.ajnr.org/lookup/doi/10.3174/ajnr.A6857
31. Monari F, Busani S, Imbrogno MG, Neri I, Girardis M, Ghirardini A, et al. Vertebral artery dissection in term pregnancy after cervical spine manipulation: a case report and review the literature. J Med Case Rep [Internet]. 2021 Dec 20;15(1):530. Available from: https://jmedicalcasereports.biomedcentral.com/articles/10.1186/s13256-021-03090-z
32. Smetanskyi V, Šefčíková A, Dörr A. Cerebral venous thrombosis during pregnancy. Ces Gynekol [Internet]. 2018;83(2):123–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29869511
33. Gazioglu S, Dinc G. Cerebral venous sinus thrombosis in pregnancy and puerperium. Acta Neurol Belg [Internet]. 2021 Aug 6;121(4):967–72. Available from: https://link.springer.com/10.1007/s13760-020-01459-3
34. Mahmoud A, Ekin U, Kania B, Shrouf A, Maroules M. Cerebral venous sinus thrombosis in pregnancy presenting with hemiplegia: A case report. Radiol Case Reports [Internet]. 2022 Oct;17(10):3713–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1930043322005520
35. Zhou B, Huang SS, Huang C, Liu SY. Cerebral venous sinus thrombosis in pregnancy: A case report. World J Clin Cases [Internet]. 2022 Jan 7;10(1):309–15. Available from: https://www.wjgnet.com/2307-8960/full/v10/i1/309.htm
36. Shigemori Y, Nonaka M, Abe H, Ogata T, Iwaasa M, Higashi T, et al. Management of Intracerebral Hemorrhage during Pregnancy and the Puerperium. Japanese J Neurosurg [Internet]. 2013;22(7):542–8. Available from: https://www.jstage.jst.go.jp/article/jcns/22/7/22_542/_article/-char/ja/
37. Vest T, Rantanen K, Verho L, Aarnio K, Korhonen A, Richardt A, et al. Etiology of intracerebral hemorrhage during pregnancy or puerperium: A nationwide study. Eur J Neurol [Internet]. 2023 Aug 11; Available from: https://onlinelibrary.wiley.com/doi/10.1111/ene.16012
38. Miller EC. Maternal Stroke Associated With Pregnancy. Contin Lifelong Learn Neurol [Internet]. 2022 Feb;28(1):93–121. Available from: https://journals.lww.com/10.1212/CON.0000000000001078
39. Fujiwara S, Ohara N, Kono T, Inui R, Imamura H, Kawamoto M, et al. Reversible Cerebral Vasoconstriction Syndrome in Early Pregnancy Treated with Endovascular Therapy. J Neuroendovascular Ther [Internet]. 2020;14(5):177–82. Available from: https://www.jstage.jst.go.jp/article/jnet/14/5/14_cr.2019-0108/_article
40. Kasuya C, Suzuki M, Koda Y, Sato H, Kashima K, Honda K, et al. A headache-free reversible cerebral vasoconstriction syndrome (RCVS) with symptomatic brain stem ischemia at late pregnancy as a rare manifestation of RCVS resolved with termination of pregnancy by semi-urgent cesarean section. Oxford Med Case Reports [Internet]. 2018 Dec 1;2018(12). Available from: https://academic.oup.com/omcr/article/doi/10.1093/omcr/omy101/5194304
41. James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and Risk Factors for Stroke in Pregnancy and the Puerperium. Obstet Gynecol [Internet]. 2005 Sep;106(3):509–16. Available from: http://journals.lww.com/00006250-200509000-00011
42. Miller EC, Vollbracht S. Neurology of Preeclampsia and Related Disorders: an Update in Neuro-obstetrics. Curr Pain Headache Rep [Internet]. 2021 Jun 7;25(6):40. Available from: https://link.springer.com/10.1007/s11916-021-00958-z
43. Verho L, Tikkanen M, Äyräs O, Aarnio K, Rantanen K, Korhonen A, et al. Pregnancy‐associated stroke and the recurrence of stroke and other complications in subsequent pregnancies: Population‐based retrospective cohort study. BJOG An Int J Obstet Gynaecol [Internet]. 2023 Oct 23;130(11):1421–9. Available from: https://obgyn.onlinelibrary.wiley.com/doi/10.1111/1471-0528.17503
44. Ijäs P. Trends in the Incidence and Risk Factors of Pregnancy-Associated Stroke. Front Neurol [Internet]. 2022 Apr 11;13. Available from: https://www.frontiersin.org/articles/10.3389/fneur.2022.833215/full
45. Katsafanas C, Bushnell C. Pregnancy and stroke risk in women. Neurobiol Dis [Internet]. 2022 Jul;169:105735. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0969996122001279
46. Doiron T, Ramseyer A, Phelps EN, Williams AJ, Teal LN, Hollenbach LL, et al. Pregnancy-Related Stroke: A Review. Obstet Gynecol Surv [Internet]. 2022 Jun;77(6):367–78. Available from: https://journals.lww.com/10.1097/OGX.0000000000001039
47. Ladhani NNN, Swartz RH, Foley N, Nerenberg K, Smith EE, Gubitz G, et al. Canadian Stroke Best Practice Consensus Statement: Acute Stroke Management during pregnancy. Int J Stroke [Internet]. 2018 Oct 18;13(7):743–58. Available from: http://journals.sagepub.com/doi/10.1177/1747493018786617
48. Elgendy IY, Gad MM, Mahmoud AN, Keeley EC, Pepine CJ. Acute Stroke During Pregnancy and Puerperium. J Am Coll Cardiol [Internet]. 2020 Jan;75(2):180–90. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109719384621
49. Yuyun MF, Sliwa K, Kengne AP, Mocumbi AO, Bukhman G. Cardiovascular Diseases in Sub-Saharan Africa Compared to High-Income Countries: An Epidemiological Perspective. Glob Heart [Internet]. 2020 Feb 12;15(1):15. Available from: https://globalheartjournal.com/article/10.5334/gh.403/
50. Liu S, Chan WS, Ray JG, Kramer MS, Joseph KS. Stroke and Cerebrovascular Disease in Pregnancy. Stroke [Internet]. 2019 Jan;50(1):13–20. Available from: https://www.ahajournals.org/doi/10.1161/STROKEAHA.118.023118
51. Gebremariam SA, Yang HS. Types, risk profiles, and outcomes of stroke patients in a tertiary teaching hospital in northern Ethiopia. eNeurologicalSci [Internet]. 2016 Jun;3:41–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2405650216300107
52. Shenkutie Greffie E. Risk Factors, Clinical Pattern and Outcome of Stroke in a Referral Hospital, Northwest Ethiopia. Clin Med Res [Internet]. 2015;4(6):182. Available from: http://www.sciencepublishinggroup.com/journal/paperinfo?journalid=151&doi=10.11648/j.cmr.20150406.13
53. Lecoffre C, de Peretti C, Gabet A, Grimaud O, Woimant F, Giroud M, et al. National Trends in Patients Hospitalized for Stroke and Stroke Mortality in France, 2008 to 2014. Stroke [Internet]. 2017 Nov;48(11):2939–45. Available from: https://www.ahajournals.org/doi/10.1161/STROKEAHA.117.017640
54. Karjalainen L, Tikkanen M, Rantanen K, Aarnio K, Korhonen A, Saaros A, et al. Stroke in Pregnancy and Puerperium. Neurology [Internet]. 2021 May 25;96(21):e2564–75. Available from: https://www.neurology.org/lookup/doi/10.1212/WNL.0000000000011990
55. Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MSV. Risk of a Thrombotic Event after the 6-Week Postpartum Period. N Engl J Med [Internet]. 2014 Apr 3;370(14):1307–15. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1311485
56. Kittner SJ, Stern BJ, Feeser BR, Hebel JR, Nagey DA, Buchholz DW, et al. Pregnancy and the Risk of Stroke. N Engl J Med [Internet]. 1996 Sep 12;335(11):768–74. Available from: http://www.nejm.org/doi/abs/10.1056/NEJM199609123351102
57. Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina E V. Hypertensive Disorders and Pregnancy-Related Stroke. Obstet Gynecol [Internet]. 2015 Jan;125(1):124–31. Available from: https://journals.lww.com/00006250-201501000-00021
58. Enomoto N, Tanaka H, Katsuragi S, Hayata E, Hasegawa J, Nakata M, et al. Pregnancy‐associated hemorrhagic stroke: A nationwide survey in Japan. J Obstet Gynaecol Res [Internet]. 2021 Jun 5;47(6):2066–75. Available from: https://obgyn.onlinelibrary.wiley.com/doi/10.1111/jog.14786
59. Sukhostavets N. Psycho-rehabilitation adaptation of pregnant women and mothers in the postpartum period who experienced traumatic events during the war. FM [Internet]. 2022 Sep. 30 [cited 2023 Jan. 14];1(3):4-12. Available from: https://futurity-medicine.com/index.php/fm/article/view/11
CONFLICT OF INTEREST
All authors declare no conflict of interest.
FUNDING
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. АР19678324).
AUTHORSHIP CONTRIBUTION:
Conceptualization: Zhanar Kypshakbayeva, Almagul Kurmanova.
Data curation: Damilya Salimbayeva, Gaukhar Kurmanova.
Formal analysis: Aizhan Turekhanova, Gaukhar Kurmanova.
Acquisition of funds: Zhanar Kypshakbayeva.
Research: All authors.
Methodology: Almagul Kurmanova, Damilya Salimbayeva.
Project management: Zhanar Kypshakbayeva.
Resources: Almagul Kurmanova, Damilya Salimbayeva.
Software: Aizhan Turekhanova.
Supervision: Zhanar Kypshakbayeva.
Validation: Gaukhar Kurmanova, Aizhan Turekhanova.
Display: Madina Khalmyrsayeva.
Drafting - original draft: Almagul Kurmanova, Damilya Salimbayeva.
Writing - proofreading and editing: All authors.