REVIEW
Pseudomonas aeruginosa: A Persistent Pathogen and Current Approaches to Treatment- Microbiology
Pseudomonas aeruginosa: un patógeno persistente y enfoques actuales de tratamiento microbiológico
Nitish Kumar1 ![]() *,
Vasundhara2, Sandeep Kumar Chavan3
*,
Vasundhara2, Sandeep Kumar Chavan3 ![]()
1Jaipur National University, School of Life and Basic Sciences, Jaipur, India.
2Teerthanker Mahaveer University, Department of Microbiology, Moradabad, Uttar Pradesh, India.
3Department of Genetics, School of Sciences, JAIN (Deemed-to-be University), Bangalore, India.
Cite as: Kumar N, Vasundhara, Kumar Chavan S. Pseudomonas aeruginosa: un patógeno persistente y enfoques actuales de tratamiento microbiológico. Salud, Ciencia y Tecnología. 2023;3:404. https://doi.org/10.56294/saludcyt2023404
Received: 25-03-2023 Reviewed: 18-04-2023 Accepted: 06-06-2023 Published: 07-06-2023
ABSTRACT
Pseudomonas aeruginosa (P. aeruginosa) represents an important bacterial pathogen, mainly because it may infect immunocompromised hosts, hospital patients, and people with cystic fibrosis (CF). Antimicrobial resistance has risen due to monitoring nosocomial P. aeruginosa infections, with tendencies toward model drug and carbapenem resistance. Some of the mechanisms of antimicrobial resistance include the downregulation of outer membrane porins, -lactamases, and multidrug efflux pumps. Toxins that be secreted and can build BioFlim (BF) are examples of virulence mechanisms. Effective therapy of infection caused by P. aeruginosa requires early delivery of the appropriate antibiotic medications, source control measures, and, where possible, prevention. Antibacterial de-escalation is supposed to be considered within patients by a positive clinical response, particularly as antibacterial susceptibilities were identified. Less common antibacterials, including Colistin, may be needed to treat multidrug-resistant P. aeruginosa, although additional anti-pseudomonal antibacterials should become accessible soon.
Keywords: Pseudomonas Aeruginosa (P. Aeruginosa); Virulence Mechanisms; Nosocomial; Colistin; Cystic Fibrosis (CF).
RESUMEN
Pseudomonas aeruginosa (P. aeruginosa) representa un importante patógeno bacteriano, principalmente porque puede infectar a huéspedes inmunodeprimidos, pacientes hospitalizados y personas con fibrosis quística (FQ). La resistencia a los antimicrobianos ha aumentado debido a la vigilancia de las infecciones nosocomiales por P. aeruginosa, con tendencia a la resistencia a los fármacos modelo y a los carbapenemes. Algunos de los mecanismos de resistencia a los antimicrobianos incluyen la regulación a la baja de las porinas de la membrana externa, las -lactamasas y las bombas de eflujo de múltiples fármacos. Las toxinas que se secretan y pueden formar BioFlim (BF) son ejemplos de mecanismos de virulencia. La terapia eficaz de la infección causada por P. aeruginosa requiere la administración precoz de los antibióticos adecuados, medidas de control de la fuente y, cuando sea posible, prevención. Se supone que la desescalada antibacteriana debe considerarse en los pacientes por una respuesta clínica positiva, en particular cuando se identificaron susceptibilidades antibacterianas. Es posible que se necesiten antibacterianos menos comunes, incluida la Colistina, para tratar la P. aeruginosa multirresistente, aunque pronto se debería poder acceder a otros antibacterianos antipseudomónicos.
Palabras clave: Pseudomonas Aeruginosa (P. Aeruginosa); Mecanismos De Virulencia; Nosocomial; Colistina; Fibrosis Quística (FQ).
INTRODUCTION
P. aeruginosa often causes infection cause within gram-negative bacteria, such as ventilator-associated wound infections, pneumonia, and Cystic Fibrosis (CF) lung infections, especially in severely ill and immunocompromised persons.(1) Especially in immunocompromised hosts, a P. aeruginosa infection considerably increases the cost of healthcare overall and may result in a severe, maybe deadly disease. Antibiotics are commonly used to treat P. aeruginosa bacteremia, although sometimes eradicating the infection may prove challenging and demand extended or extensive treatment.(2)
In nosocomial conditions, bacterial biofilm development must be avoided at all costs. Nosocomial infections caused by biofilm threaten human health and financial security since they may result in several potentially fatal illnesses. Scientists from all around the globe have developed a wide range of Metallic Nano-Particles (MNPs). At the same time, their effectiveness in treating bacterial infections, with those in strains opposing antibiotics, has been assessed. Most MNPS made of silver, copper, zinc, and gold can stop BioFilms (BF) from growing, but they have cytotoxic effects at high concentrations.(3)
In addition to soil, water, plants, human skin, and oral mucosa, P. aeruginosa is one of the most widely distributed and biochemically adaptable bacteria. For nutrition, it may also catabolize a broad range of chemical substances. The prevalence of infections rises as a result of P. aeruginosa's capacity to flourish in a range of habitats, which raises its reservoirs and exposure hazards.(4)
The variety of virulence factors at the pathogen's disposal contributes to its adaptability and flexibility, enabling P. aeruginosa to tailor its response to various environmental cues. Understanding the pathoadaptability of this pathogen requires an understanding of the massive P. aeruginosa genome, which contains a variety of genetic regulatory mechanisms, particularly including novel genomic tools that allow the evaluation of differences and similarities among P. aeruginosa populations.(5) Schematic representations of the initial infections caused by P. aeruginosa are exposed in figure 1.
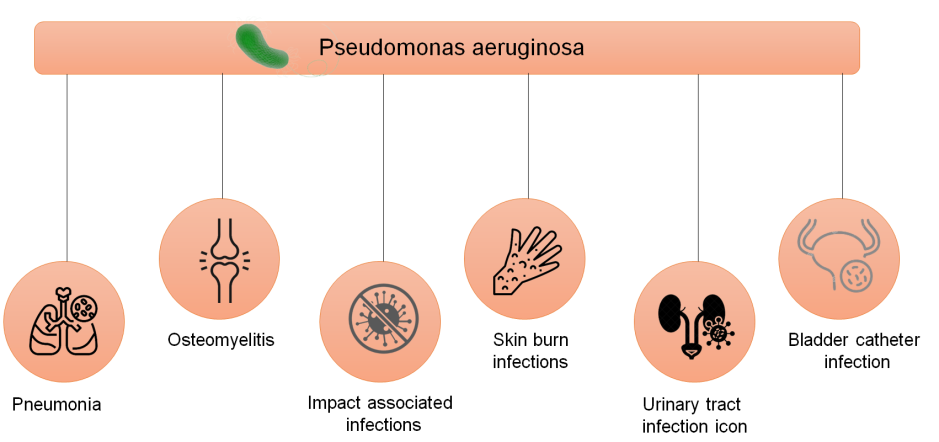
Figure 1. Pseudomonas aeruginosa's primary infections
Hospital-acquired diseases are significantly challenged by heteroresistant infections linked to P. aeruginosa. However, more research must be done on the prevalence and effects of P. aeruginosa heteroresistance in clinical settings.(6)
Population genomics studies have shown various genetic differences in patients, including diverse lineages derived via patient-to-patient transmission for transmissible strains.(7) The study(8) offers information on the manufacture and control of this exopolysaccharide and reviews findings on the involvement of alginate in P. aeruginosa-induced CF-associated lung infections. P. aeruginosa isolates from 30 individuals with chronic diseases, and underlying non-CF respiratory illnesses were examined.(9)
The author(10) described a 17-month research of a group of MDR P. aeruginosa isolates that were taken as of an infantile feminine CF patient who has Double Lung Transplantation (DLP). The study(11) examined the growth of antibiotic resistance with antimicrobial tolerance in P. aeruginosa biofilms. The activity of Poly Morpho Nuclear leukocytes (PMNs) and the interaction between tobramycin and garlic have both been studied.(12)
The study(13) focused on the strategy against bacterial biofilm development and quorum-sensing systems and evaluated the use of bacteriophages as a therapy. The research(14) was conducted because it was unknown if P. aeruginosa and subgingival periodontal pathogenic microorganisms in CF patients' lungs and oral cavities were related. The study(15) concentrated on the multiple benefits of phage treatment for P. aeruginosa biofilm reduction within medical plus in vitro studies to emphasize the therapy's broad applicability.
DEVELOPMENT
Virulence, infection, and resistance mechanisms
Attachment versus Motility
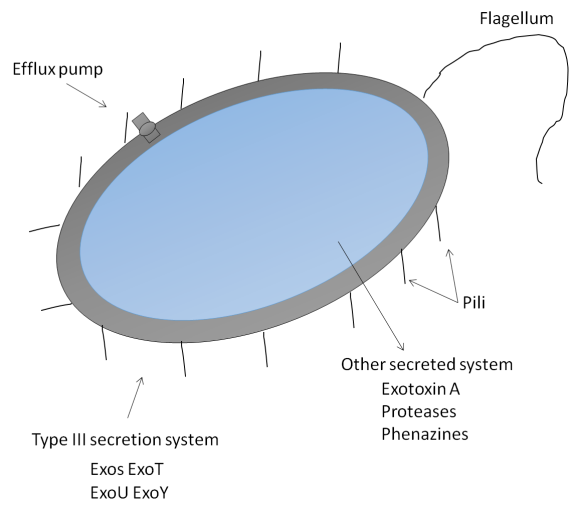
P. aeruginosa is motile and may facilitate first surface contacts thanks to its solitary flagellum. Numerous type IV cell surface pili, which are in charge of attachment to other surfaces and cell membranes, are also present in P. aeruginosa. Asia-Ganglioside M1 (aGM1), a glycolipid found on the surface of epithelial cells in the respiratory system, is one binding site. Only injured respiratory epithelium has been proven to harbor P. aeruginosa, which may be accounted for by the fact that aGM1 is mainly generated during the healing process of epithelial cells. Multiple pathogenic pathways may appear after cell surface attachment (figure 2).
|
|
|
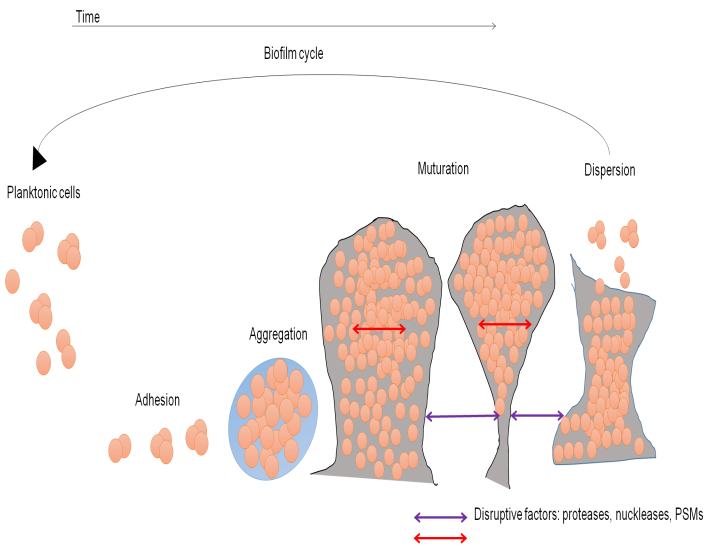
Figure 2. (a) Mechanism of P. aeruginosa virulence as well as antibiotic resistance (b) Mode of BF growth
sQuorum sensing,BF formation, and alginate secretion
The extracellular polysaccharide alginate is produced in excess by certain P. aeruginosa strains, a condition known as mucoid. Mutations in the mucA gene are common in mucoid isolates. AlgU, also known as AlgTor 22, may activate the genes responsible for producing alginate without MucA. Alginate may stop the sick host from eliminating microorganisms from its body by preventing phagocytosis, scavenging free radicals formed in macrophages, forming a substantial barrier to encourage, neutrophil chemotaxis, activating complement along with preventing phagocytosis, Alginate also seems selected necessary for the development of P. aeruginosa Biofilms (BF).
A BF is a particular kind of bacterial growth that forms from the adhesion of microcolonies to a surface and their enclosing in a biopolymer matrix. P. aeruginosa BF has been seen within the airways of CF patients, and it is widely known that biofilms on indwelling medical devices may harbor bacteria.
Type 3 Secretion System (SS)
Type 3 SS may start to function after P. aeruginosa attaches to an epithelial cell. P. aeruginosa may directly inject certain effectors protein molecules into epithelial cells via the such contact-dependent pathway, altering immune responses and causing cell damage and death. The four exoenzymes that have already been discovered, “ExoS, ExoT, ExoU, and ExoY”, are articulated within dissimilar strains with contain a variety of roles.
Out of all the exoenzymes, ExoU may be the most harmful. Improved humanity in patients with “pneumonia, sepsis, respiratory failure, and more severe illness in Ventilator-Associated Pneumonia (VAP)” have all been linked to developing the type SS in P. aeruginosa isolates.
Type 3 SS was decreased in vivo P. aeruginosa pneumonia models in mice, rabbits, and rats by delivering antibody products that target PcrV. Compared to controls, this reduced lung damage, shock, and death.
Additional Secreted Virulence Elements
Although a comprehensive analysis is beyond the purview of this study, additional P. aeruginosa virulence factors are here briefly mentioned. For further detail, the reader is directed to two exceptional recent works. Eukaryotic elongation factor 2 is inhibited by exotoxin A, which prevents protein synthesis and kills host cells.
The surfactant proteins A, D, complement, antimicrobial peptides, and immunoglobulin are only a few examples of the host immunoregulatory proteins that secreted enzymes may break down. The host cells are harmed by oxidative and pro-inflammatory processes caused by the released phenazines, which include pyocyanin, and affect the respiratory system.
Antimicrobial Resistance Mechanisms
Antibacterial resistance often manifests as target structural modification, active efflux from the cell, enzymatic breakdown, and entrance blockage. P. aeruginosa may use any of these techniques to build resistance. The reason P. aeruginosa is intrinsically resistant to antibiotics is mostly due to the shallow permeability of its outer membrane compared to other Gram-negative bacteria like Escherichia coli and efflux mechanisms.
The multimodal efflux pumps of P. aeruginosa may lead it to export antibiotics. Effective efflux pump systems need to encompass the internal with external membranes between the membranes in the periplasm since they are thought of as tripartite geometries. The P. aeruginosa genome contains as a minimum ten separate efflux pump system operons, although only the multimodal efflux pumps "MexA-MexB-OprM," "MexC-MexD-OprJ," "MexE-MexF-OprN," and "MexX-MexY-OprM" have been thoroughly described.
The overexpression of a multimodal efflux pump that can handle a variety of antibacterial substrates increases the "Mean Inhibitory Concentration (MIC)" of any medication that is vulnerable to the efflux pump (table 1). The amount of pressure is applied by selecting strains of P. aeruginosa with excessive production of these efflux pumps. Due to these properties, fluoroquinolones that have been identified as the substrate of all four pumps for efflux listed above may provide unique challenges.
|
Table 1. Multidrug efflux pumps from P. aeruginosa with antibacterial substrates |
|||
|
MexX-MexY-OprM |
MexA-MexB-OprM |
MexE-MexF-OprN |
MexC-MexD-OprJ |
|
Sulbactam |
Chloramphenicol |
Levofloxacin |
Gentamicin |
|
Chloramphenicol |
Aztreonam |
Cefepime |
Amikacin |
|
Norfloxacin |
Cefuroxime |
Erythromycin |
Erythromycin |
|
Tetracycline |
Faropenem |
|
Faropenem |
|
Ciprofloxacin |
Carbenicillin |
Cefuroxime |
Cefepime |
|
Clavulanic acid |
Cefotaxime |
Chloramphenicol |
Cefotaxime |
|
Levofloxacin |
Ceftazidime |
Ciprofloxacin |
Ciprofloxacin |
|
Norfloxacin |
Clavulanic acid |
|
Clavulanic acid |
|
Norfloxacin |
Cefuroxime |
Erythromycin |
Erythromycin |
|
Trimethoprim |
Ciprofloxacin |
Nafcillin |
Levofloxacin |
|
MexE-MexF-OprN |
MexA-MexB-OprM |
MexC-MexD-OprJ |
MexX-MexY-OprM |
Therapy for P. aeruginosa infection
Procedures and Prevention Measures for Infection Control
When possible, P. aeruginosa infections should be avoided to manage them. There are several accounts of nosocomially acquired P. aeruginosa infection outbreaks in the medical literature, and some cases may be linked to hospital staff members' chronic carriage conditions.
Finding At-Risk Individuals and Gathering Cultural Data
Healthcare-Associated Pneumonia (HCAP), Hospital-Acquired Pneumonia (HAP), or VAP, Intensive Care Units (ICU) contracting infections, neutropenic sepsis brought on by chemotherapy, acute leukemia, or AIDS, in addition to CF with rapidly increasing bronchiectasis, are some of the causes for using anti-pseudomonal antibacterial treatment. The fact that antibacterial regimens frequently undergo modification when an interfering bacterium is identified—ideally, before antibiotic treatment, if this can be done on time—highlights the need to acquire cultures.
Source control with immediate antimicrobial treatment
Antibacterial treatment shouldn't be deferred, especially in the highly ill, to deliver the proper antibacterials to the sickest patients—those with septic shock and severe sepsis within one hour. For the effective treatment of any ailment, source management is often necessary. Any early or persistent infections that may be treated with drainage, debridement, or removal should be thoroughly examined in patients.
After initial stabilization and the administration of antibiotics, abscesses with empyemas should be eliminated, contaminated surgical instruments should be removed, and additional sepsis causes should be treated with the support of qualified specialists.
The Advantages of Effective Initial Therapy
It is well established that inappropriate initial empirical antibiotic dosing hurts patient outcomes. It was shown in a recent retrospective review that P. aeruginosa bloodstream infections needed the proper therapy. When sensitivity information was available, patients who did not get at least one drug that a bloodstream P. aeruginosa strain was sensitive to or whose first antibiotic treatment was insufficient had considerably higher mortality rates.
Treatment of P. aeruginosa: Combination vs Monotherapy
Treatment for P. aeruginosa bacteremia was split into practical and conclusive approaches when it was shown that an appropriate scientific mixture Anti-Pseudomonal Therapy (APT) be linked utilizing decreased humanity in a single month than an acceptable empirical anti-pseudomonal monotherapy. There was no difference in the mortality rates between a good standard combination treatment and an excellent traditional monotherapy.
The theory of antibiotic de-escalation
Treatment for P. aeruginosa bacteremia was split into empirical and definitive techniques when it was shown that a sufficient practical combination of APT was linked with reduced humanity in a single month than an acceptable experimental Anti-Pseudomonal Mono-Therapy (APMT). In addition, individuals with recurring infections and those who had previously had 15 days of antibiotic therapy are additional probable to multiresistant bacteria.
Recommendations for Specific Doses in P. aeruginosa
Only sometimes are comparative trials of alternative dosage administration methods for the same antibacterial drug available. The pharmacokinetic/pharmacodynamic properties of the used antibacterial agents possess an impact on several recommendations for dosage administration. These characteristics vary amongst antimicrobial agent groups.
The “American Thoracic Society (ATS) with the Infectious Disease Society of America (IDSA)” has developed consensus recommendations for the treatment of nosocomial patients with late-onset pneumonia caused by a common bacterium, P. aeruginosa.
|
Table 2. The first empirical antibiotic therapy for P. aeruginosa pneumonia |
|
|
Antibacterial |
Dosage |
|
One of the following: |
|
|
Cefepime |
IV1–2gevery8–12hours |
|
Piperacillin/tazobactam |
IV4,5gevery6hours |
|
Imipenem cilastatin |
IV500mgevery6hoursor1gevery8hours |
|
Ceftazidime |
IV2gevery8hours |
|
Aztreonam |
IV2gevery8hours |
|
Meropenem |
IV1gevery8hours |
|
Plus one of the following: |
|
|
Gentamicin |
IV7mg/kgoncedailyc |
|
Tobramycin |
IV7mg/kgoncedailyc |
Table 2 lists acceptable anti-pseudomonal medications along with suggested dosages. Typically, therapy shouldn't go more than 7-8 days if there is a robust clinical response and standard lung architecture.
Alternatives to Antimicrobials
Colistin
Due to the growing issue of MDR Gram-negative bacteria that might simply respond to these treatments whereas exhibiting a variety of resist techniques to exclude the employ of other drugs, the usage of colistin has grown lately. Intravenous Colistin's efficacy for treating severe infections caused by MDR bacteria is good, given that its use is sometimes necessary due to the absence of adequate substitutes. Due to the issue with MDR Gram-negative bacteria that might respond to this treatment even as demonstrating a range of resistance mechanisms that exclude alternate therapy, colistin usage has grown. Given that the lack of adequate alternatives often necessitates its use, intravenous Colistin seems effective in treating severe infections by MDR pathogens.
Doripenem
“Doripenem is a brand-new 1-methyl-carbapenem” which is stable against -lactamases with resistance to inactivation by renal dihydro peptidases. All Gram-negative bacteria and Gram-positive bacteria, with P. aeruginosa, are susceptible to doripenem in vitro, except MRSA and a few Enterococcus species. Studies comparing doripenem to meropenem or imipenem showed that doripenem had a more significant in vitro anti-pseudomonal effect.
P. aeruginosa Treatment for Cystic Fibrosis Patients
The best locations to provide long-term care for CF patients are specialized care facilities, and P. aeruginosa therapy at these facilities often entails ongoing follow-up and sputum culture monitoring. This section intends to inform non-CF experts on the approach used at these institutions to treat persistent CF airway infections, including internists, pulmonologists, along with intensivists.
Initial Colonization Treatment
Most CF patients will ultimately get a respiratory tract infection caused by P. aeruginosa, and it is thought that once this happens, it is almost tough to get rid of. A predominance of P. aeruginosa mucoid phenotypes characterizes the chronic disease. When P. aeruginosa is recognized on top of observation sputum or throat swab cultures, a strong antibiotic drug that primarily targets P. aeruginosa is attempted. These trials have shown that, when compared to untreated controls, this method reduces the likelihood of acquiring a long-term infectious illness, improves lung function, and cuts down on the number of hospital days. It is uncertain if this course of action will be effective in the long run for patients who relapse and need therapy.
Chronic Stimulatory Therapy
When P. aeruginosa is repeatedly cultivated from CF patients' sputum for six months, the condition is commonly referred to as chronic P. aeruginosa infection. Once a persistent infection has been established, trials of planned intermittent antibiotic treatment have been conducted to see if this approach would affect the clinical course of the condition.
Acute respiratory illnesses
Acute CF respiratory exacerbations have been treated with greater antibacterials as well as shorter intervals between doses than pneumonia in non-CF patients based on pharmacokinetic studies demonstrating enhanced drug authorization along with shortened removal half-life in CF patients. P. aeruginosa mucoid plugs stop the CF airway from absorbing the antibiotics. Antibiotic treatment frequently lasts from 14 to 21 days, depending on the seriousness of the symptom plus the prognosis.
For moderate pulmonary symptoms, oral ciprofloxacin at an amount of 29 mg/kg daily, split into a proposal or tid quantity delivery interval is often utilized. Intravenous antibiotics are used to treat more serious illnesses. Inhaled anti-pseudomonal medications are seldom utilized to treat acute pulmonary exacerbations despite being an essential part of CF patients' maintenance regimens owing to a lack of evidence.
|
Table 3. Acute exacerbations of pulmonary CF are managed with anti-pseudomonal regimens |
|
|
Antibacterial |
Dosage |
|
One of the following: |
|
|
Cefepime |
IV2gevery8hours |
|
Ceftazidime |
IV2gevery8hours |
|
Meropenem |
IV1gevery8hours |
|
Plus: |
|
|
Tobramycin |
IV3mg/kgevery8hoursb |
The majority of CF clinicians advise administering two intravenous anti-pseudomonal drugs simultaneously to treat moderate to severe acute pulmonary exacerbations of CF despite the lack of compelling evidence.
Table 3 displays the typical treatment plans for adult CF patients at Washington University in Saint Louis. When selecting these first antibacterials, the most recent sputum culture results are often employed, and newly discovered sputum culture discoveries may alter them. Additionally, patients who have tested positive for various bacterial illnesses may get treatment designed mainly to ward against these strains.
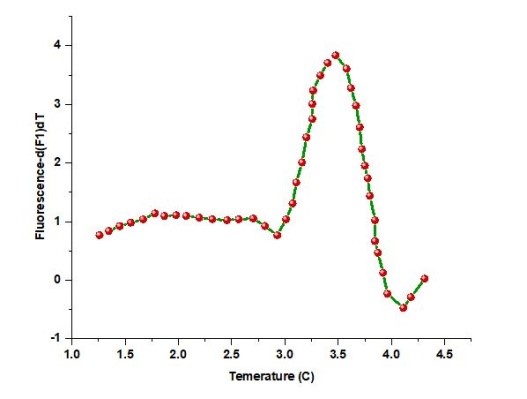
Real-time, fluorescence-based quantitative PCR
The technique made it possible to find P. aeruginosa in CF patients more quickly. It created a real-time PCR technique that targeted the gyrB gene by utilizing melting curve analysis (MCA). This technique could easily differentiate P. aeruginosa from other microorganisms, as shown in Figure 3. Using 224 Gram-negative bacterial samples, a performance comparison of the real-time PCR approach and the Vitek detection system revealed that this method could successfully distinguish P. aeruginosa from other Gram-negative bacteria within 3 hours. A duplex real-time Polymerase Chain Reaction (PCR) test was developed against the genes ecfX and gyrB to address false-positive and false-negative concerns in identifying Pseudomonas aeruginosa.
|
|
|
Figure 3. Gram-negative bacilli MCA (a) Positive samples for P. aeruginosa had firm peaks with a consistent (b) Non-P. aeruginosa samples did not exhibit a peak at 87,9 °C
By contrasting the specificity and sensitivity of different genes, it was shown that real-time multiplex PCR could successfully resolve the conflict between sensitivity and specificity and identify P. aeruginosa from a mixed bacterial sample. An important pathogen in pneumonia and respiratory tract infections was also detected using real-time fluorescence-based PCR, which was a very sensitive, powerfully rapid, extensively applicable, and prospectively detectable method.
CONCLUSION
P. aeruginosa is a dangerous bacterial pathogen known to cause CF patients' sino-pulmonary infections, nosocomial infections, and infections in immunocompromised hosts. Due to its many virulence features and antibacterial resistance mechanisms, P. aeruginosa has recently seen a surge in the prevalence of antibacterial resistance. Wherever possible, steps should be taken to avoid P. aeruginosa infections. Patients at high risk should also get the necessary first antimicrobial therapy. If the criminal detach with identified sensitivities be present and the disease is acute and uncomplicated, the de-escalation of antibacterials to a single medicine other than an aminoglycoside is supposed to be working in the context of a successful clinical response. People with CF are often treated for P. aeruginosa infections by CF specialists, while non-CF doctors may be able to help if their condition is severe. To treat MDR P. aeruginosa, doctors need to be knowledgeable about older antibiotics like Colistin, antibiotics that may be breathed for respiratory tract infections, and antibiotics that will probably be widely available shortly, such as doripenem.
REFERENCES
1. Hu Y, Zhu K, Jin D, Shen W, Liu C, Zhou H, Zhang R. Evaluation of IR Biotyper for carbapenem-resistant Pseudomonas aeruginosa typing and its application potential for investigating nosocomial infection. Front Microbiol. 2023;14.
2. Ng QX, Ong NY, Lee DYX, Yau CE, Lim YL, Kwa ALH, Tan BH. Trends in Pseudomonas aeruginosa (P. aeruginosa) Bacteremia during the COVID-19 Pandemic: A Systematic Review. Antibiotics. 2023;12(2):409.
3. MubarakAli D, Arunachalam K, Lakshmanan M, Badar B, Kim JW, Lee SY. Unveiling the Anti-Biofilm Property of Hydroxyapatite on Pseudomonas aeruginosa: Synthesis and Strategy. Pharmaceutics. 2023;15(2):463.
4. Wood SJ, Kuzel TM, Shafikhani SH. Pseudomonas aeruginosa: Infections, Animal Modeling, and Therapeutics. Cells. 2023;12(1):199.
5. Mendes OR. The challenge of pulmonary Pseudomonas aeruginosa infection: How to bridge research and clinical pathology. In: Viral, Parasitic, Bacterial, and Fungal Infections. Academic Press; 2023. p. 591-608.
6. Chen Z. Mechanisms and Clinical Relevance of Pseudomonas aeruginosa Heteroresistance. Surg Infect. 2023.
7. Winstanley C, O’Brien S, Brockhurst MA. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 2016;24(5):327-337.
8. May TB, Shinabarger D, Maharaj ROMILA, Kato J, Chu L, DeVault JD, Roychoudhury SIDDHARTHA, Zielinski NA, Berry A, Rothmel RK. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev. 1991;4(2):191-206.
9. Maciá MD, Blanquer D, Togores B, Sauleda J, Pérez JL, Oliver A. Hypermutation is a key factor in developing multiple-antimicrobial resistance in Pseudomonas aeruginosa strains causing chronic lung infections. Antimicrob Agents Chemother. 2005;49(8):3382-3386.
10. Rojas LJ, Yasmin M, Benjamino J, Marshall SM, DeRonde KJ, Krishnan NP, Perez F, Colin AA, Cardenas M, Martinez O, Pérez-Cardona A. Genomic heterogeneity underlies multidrug resistance in Pseudomonas aeruginosa: A population-level analysis beyond susceptibility testing. PLoS One. 2022;17(3):e0265129.
11. Ciofu O, Tolker-Nielsen T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics. Front Microbiol. 2019;10:913.
12. Bjarnsholt T, Jensen PØ, Rasmussen TB, Christophersen L, Calum H, Hentzer M, Hougen HP, Rygaard J, Moser C, Eberl L, Høiby N. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology. 2005;151(12):3873-3880.
13. Savoia D. New perspectives in the management of Pseudomonas aeruginosa infections. Future Microbiol. 2014;9(7):917-928.
14. Rivas Caldas R, Le Gall F, Revert K, Rault G, Virmaux M, Gouriou S, Héry-Arnaud G, Barbier G, Boisramé S. Pseudomonas aeruginosa and periodontal pathogens in the oral cavity and lungs of cystic fibrosis patients: a case-control study. J Clin Microbiol. 2015;53(6):1898-1907.
15. Chegini Z, Khoshbayan A, Taati Moghadam M, Farahani I, Jazireian P, Shariati A. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: A review. Ann Clin Microbiol Antimicrob. 2020;19:1-17.
FINANCING
No financing.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION:
Conceptualización: Nitish Kumar, Vasundhara, Sandeep Kumar Chavan.
Investigación: Nitish Kumar, Vasundhara, Sandeep Kumar Chavan.
Redacción – revisión y edición: Nitish Kumar, Vasundhara, Sandeep Kumar Chavan.