ORIGINAL ARTICLE
A novel optimization of hybrid feature selection algorithms for image classification technique using RBFNN and MFO
Novedosa optimización de algoritmos híbridos de selección de características para la técnica de clasificación de imágenes mediante RBFNN y MFO
Kumar Siddamallappa Ujjappanahalli1 ![]() *, Vijay Ramnath Sonawane1
*, Vijay Ramnath Sonawane1 ![]() *, Nisarg Gandhewar1
*, Nisarg Gandhewar1 ![]() *
*
1Dr.A.P.J. Abdul Kalam University, Department of Computer Science & Engineering, Indore, India.
Cite as: Siddamallappa U K, Sonawane VR, Gandhewar N. A novel optimization of hybrid feature selection algorithms for image classification technique using RBFNN and MFO. Salud Cienc. Tecnol. 2022;2(S2):241. https://doi.org/10.56294/saludcyt2022241
Submitted: 04-11-2022 Revised: 30-11-2022 Accepted: 17-12-2022 Published: 31-12-2022
ABSTRACT
A brain tumor develops when abnormal cells in brain tissue multiply uncontrollably. For radiologists, finding and categorizing tumors manually has become a demanding and time-consuming task. When radiologists or other clinical professionals need to extract an infected tumor area from an MR picture, they have to go through a lengthy and laborious process. To improve performance and simplify the segmentation process, we investigate the FCM-predicted picture segmentation techniques in this study. In addition, classifiers for automating the detection and reclassification of encephalon tumors receive input consisting of critical information obtained from each segmented tissue. We have assessed, verified, and demonstrated the experimental efficacy of the proposed method. The purpose of this research was to develop a novel MFO (Moth-Flame Optimization) based LLRBFNN model for the automatic detection and classification of benign and malignant brain tumors. In order to alleviate the burden of manually detecting encephalon cancers from MR images, the suggested LLRBFNN model parameters are improved via MFO training. The Modified FCM method removes outlying nodes from the LLRBFNN model, and the MFO algorithm keeps the current of node centres in the aforementioned model. The proposed MFO-LLRBFNN model was evaluated alongside the Decision Tree and the Random Forest. To prove the reliability of this model, an MFO-based LLWNN (Local Linear Wavelet Neural Network) model for autonomously detecting brain cancers was presented. We extracted features from MR images using the MFCM (modified fuzzy C-Means) segmentation algorithm and the GLCM (Gray Level Co-occurrence Matrix) technique.
Keywords: MFO; Brain Tumor; Modified Fuzzy C-Means; MR Images; LLRBFNN.
RESUMEN
Un tumor cerebral se desarrolla cuando las células anormales del tejido cerebral se multiplican sin control. Para los radiólogos, encontrar y clasificar los tumores manualmente se ha convertido en una tarea exigente y lenta. Cuando los radiólogos u otros profesionales clínicos necesitan extraer una zona tumoral infectada de una imagen de RM, tienen que pasar por un proceso largo y laborioso. Para mejorar el rendimiento y simplificar el proceso de segmentación, en este estudio investigamos las técnicas de segmentación de imágenes predichas por FCM. Además, los clasificadores para automatizar la detección y reclasificación de tumores de encéfalo reciben una entrada consistente en información crítica obtenida de cada tejido segmentado. Hemos evaluado, verificado y demostrado la eficacia experimental del método propuesto. El propósito de esta investigación fue desarrollar un novedoso modelo LLRBFNN basado en MFO (Moth-Flame Optimization) para la detección y clasificación automática de tumores cerebrales benignos y malignos. Con el fin de aliviar la carga que supone la detección manual de cánceres encefálicos a partir de imágenes de RM, los parámetros del modelo LLRBFNN sugerido se mejoran mediante el entrenamiento MFO. El método FCM modificado elimina los nodos periféricos del modelo LLRBFNN, y el algoritmo MFO mantiene la corriente de centros de nodos en dicho modelo. El modelo MFO-LLRBFNN propuesto se evaluó junto con el Árbol de decisión y el Bosque aleatorio. Para demostrar la fiabilidad de este modelo, se presentó un modelo LLWNN (Local Linear Wavelet Neural Network) basado en MFO para la detección autónoma de cánceres cerebrales. Se extrajeron características de imágenes de RM utilizando el algoritmo de segmentación MFCM (fuzzy C-Means modificado) y la técnica GLCM (Gray Level Co-occurrence Matrix).
Palabras clave: MFO; Neoplasias Encefálicas; Modified Fuzzy C-Means; Imagen por Resonancia Magnética; LLRBFNN.
INTRODUCTION
Dimensionality reduction is required before extracting the most informative features from a medical image.(3) Using this feature extraction technique will be quite helpful when working with large picture sizes, and a reduced feature demonstration may be necessary to swiftly finish tasks like MRI image matching and retrieval.(1)
Using novel segmentation approaches and classifiers,(4,5) this study aims to detect and categorize brain cancers. Inaccurate tumor detection has been a problem with current segmentation methods such as wavelet transform segmentation, KMeans algorithm segmentation, and FCM-based segmentation. Sound, artifacts, and inconsistent intensities are all problems for MRIs. These problems meant that the traditional method of image segmentation(2) was not yielding reliable results. First, we presented an improved EnFCM for better segmentation results. A new mathematical model with adjusted parameters for the Modified FCM's intensity and grayscale has been introduced. Furthermore, the calculation time and noise have been reduced by introducing the fuzzy factor and changing the spatial parameter in the EnFCM (Modified FCM) cost function.
Rician noise in MRI images of brain tumors was not diminished despite the fact that the fuzzy factor was used as the cost function of MFCM for noise reduction. It has been suggested that a modified Fuzzy C Means (FCM) can improve Rician noise reduction and tumor diagnosis from an MRI. Mathematical analyses have been developed for the exponential enhancement of the fuzzy factor of the cost function, but they have not been successful in removing Gaussian noise from MRIs.
The segmentation and noise reduction performance of MRI images have been improved by implementing a fast and robust FCM to improve robustness against noise. For better segmentation accuracy, FCM uses median filtering to lower background noise. To minimize salt-and-pepper, Gaussian, and continuous noise by more than 30 %, the FCM requires more parameters and has limited success.
A new MFO is presented in this research (Moth-Flame Optimization). The expected LLRBFNN model for classifying encephalon tumors. Magnetic Resonance (MR) images were segmented using the modified fuzzy c means method (MFCM), and features were removed using the generalized linear classifier fusion method (GLCM). The goal of this research is to use hybrid models and algorithms to classify and segment encephalon tumors in MR images. The collected features were used to inform the suggested LLRBFNN model for benign and malignant tumor migration, which was developed using MFO predictions.
Related work
Literature demonstrates numerous strategies for detecting brain tumors. Charutha and Jayashree made a proposal concerning the malignant Brain Tumor. Their analysis demonstrates that PET and MRI imaging play a significant role in the medical imaging of brain tumors.(6)
The work focused on developing a noise-reduction method that recovers useful pictures by means of gray-level co-occurrence matrix (GLCM) features.(7,8) In order to simplify the process and boost efficiency, we used Discrete Wavelet Transform (DWT)-based segmentation to analyze brain tumors.
The suggested method employs morphological filtering, following segmentation, to effectively eradicate background noise. At last, an MRI image classifier based on a probabilistic neural network was developed. It presented a deep cascaded neural network-based automatic segmentation method for Brain vivo Gliomas using MRI data. Separate networks, one for pinpointing tumors and another for classifying cells within tumors, make up this larger network.(9,10) The results from this strategy are extremely plausible. The main strength of this method is that it can merge different parts of tumor images, which is something that other approaches to this problem lack.
To routinely identify brain tumors, introduce a hybrid replica PSO- LLRBFNN Algorithm that achieves an accuracy of 98 % for the ADNI dataset.(11,12)
A method for encephalon tumor segmentation was suggested based on a hybrid type of approach using FCM, and they reached 90 % noise level precision.(13) When compared to first-order statistical characteristics,(14) it was found that using a wavelet transform and SVM's technique resulted in better prognoses and enhanced clinical factors such as tumor volume, and tumor stage.(15) Using PCA and SVMs, a 94 % relegation accuracy (SVM) was obtained.(16)
Moreover, Cui et al. created a localized fuzzy clustering using geodata, claiming an accuracy of 85-95 percent. With the introduction of an active contour model,(17,18 )a method for medical image segmentation has been developed that can compensate for intensity inhomogeneity.
The use of the Gaussian mixture model (GMM) with MR images for automatic feature extraction and PCA to improve the GMM feature extraction was investigated as well.(19,20) Those studies reported a precision of 93,2 %, a sensitivity of 91,6 %, and a specificity of 97,8 % for brain tumor classification from 3D MR images using an extreme learning machine.(21,22)
METHODS
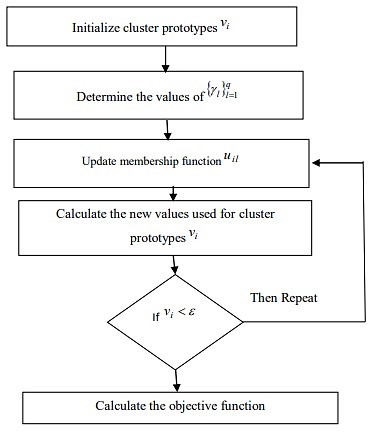
Our research focuses on the classification of medical images using clustering techniques. The job flow was done in three stages. The study task is divided into the following steps: (i) The segmentation was performed using the MFCM algorithm, and feature extraction was performed using the GLCM feature extraction technique. Additionally, the second phase (ii) provided the eliminated features as input to the MFO-RBFNN (iii) performance evaluation. The Flow Chart of our Proposed Work is shown in Figure 1.
|
|
|
To significantly reduce the quantity of estimation conducted during the segmentation process, the MFCM algorithm was introduced to accelerate the gray level picture segmentation method. Modified Fuzzy C-means Algorithm is shown in Figure 2.
Inspired by the transverse orientation navigation techniques of moths, teams like the ones of Huang and Clark, proposed a Moth-Flame Optimization algorithm.(23,24) In this algorithm, the term "population" refers to a collection of moths, and it is believed that flame is the finest treatment for every moth. This algorithm falls under the same principle as PSO. In PSO, each particle's 'p-best' is considered to be its personal best. Similarly, this moth-flame includes one flame per moth, which is regarded as its optimal placement.(25,26) During the iteration, this flame will be updated if a superior option becomes available. Pseudo code of MFO is shown in Figure 3.
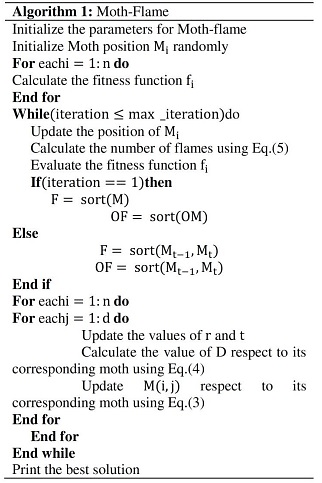
Figure 3. Pseudo code of MFO
The following describes the rationale for initiating the model.
1. When modelling samples are limited, the models enable economical interpolation in high-dimensional domains.
2. When LLRBFN and RBFN are co-expressed, LLRBFN requires fewer neurons than RBFN.
3. Because the nodes in the input and hidden layers are equivalent, the total number of nodes required is minimized.
The RBFNN converges faster and requires fewer neurons than the Multilayer Perceptron (MLP). Local Linear Radial Basis Function Neural Networks (LLRBFN) have been proposed as a way to improve RBFNN performance.
SIMULATION RESULTS
The features were extracted using the GLCM approach, and image segmentation was performed using the Modified FCM technique. The Modified FCM segmentation accuracy outperformed previous approaches in terms of accuracy and noise reduction capability. The proposed MFO-RBFNN model outperformed previously used models in terms of classification accuracy (Figure 4).

Figure 4. MFO-RBFNN
Table 1 compares the performance of several classifiers.
|
Table 1. Performance Comparison between Various Classifiers |
|||||||
|
Classifiers |
Accuracy |
Recall |
Precision |
F1-Score |
Time to train |
Time to predict |
Total time |
|
RBFNN |
96,49 % |
96,52 % |
96,49 % |
96,47 % |
0,01 |
206,91 |
206,91 |
|
DECISION TREE |
91,23 % |
91,23 % |
91,61 % |
91,30 % |
0,01 |
0,00 |
0,01 |
|
RANDOM FOREST |
95,41 % |
95,61 % |
95,61 % |
95,60 % |
0,14 |
0,02 |
0,15 |
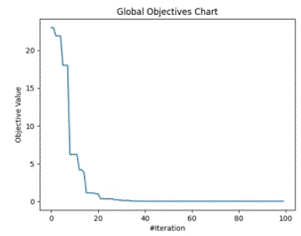
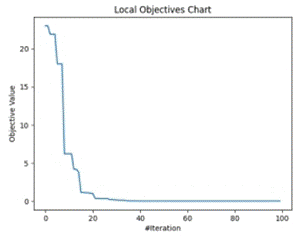
Figure 5 shows the trajectory of finding optimum points by clusters of optimizers at local level ((a) - inside cluster) and at global level ((b)-dataset level). MFO results were based on Calculation of Best Feature.
|
|
|
|
(a) |
(b) |
Figure 5. Global and Local objective value
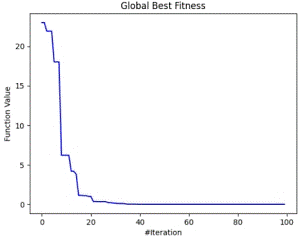
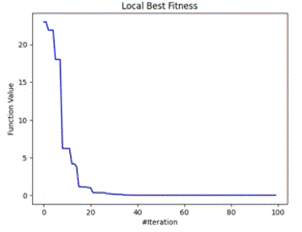
Figure 6 shows the trajectory of fitness between two optimum points by clusters of optimizers at local level ((a) - inside cluster) and at global level ( (a) - dataset level).
|
|
|
|
(a) |
(b) |
Figure 6. Global and Local Best Fitness
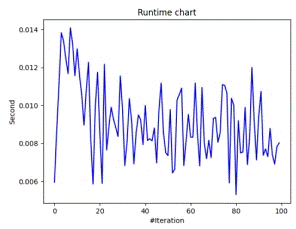
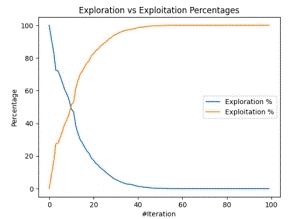
Figure 7(a) shows the time taken by the optimizer to find an optimum point per epoch, while Figure 7(b) shows a trajectory comparison b/w exploitation and exploration between two optimum points by clusters of optimizers.
|
|
|
|
(a) |
(b) |
Figure 7. Runtime and Exploration/Exploitation Chart
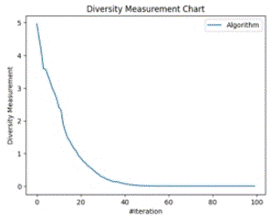
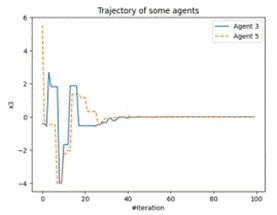
Figure 8 (a) shows how diverse one cluster’s optimal point finding trajectory is from another cluster’s optimal point finding trajectory, while Figure 8 (b) depicts what is the trajectory of the cluster in finding optimum points over the entire epoch run.
|
|
|
|
(a) |
(b) |
Figure 8. Density Measurement and Agent-Based Trajectory Chart
We also Selected 12 Features based on Optimum Points: mean radius, mean fractal dimension, area error, smoothness error, compactness error, fractal dimension error, worst texture, worst perimeter, worst smoothness, worst concavity, worst symmetry, worst fractal dimension.
Results after Applying Optimized Data on Random Forrest Classifier
Subset accuracy: 0,9949122807017544
Total Time: - 0,008975982666015625
CONCLUSION
The proposed MFO-LLRBFNN model could distinguish between cancerous and benign tumors with high accuracy. In addition, the MFCM method was used for picture segmentation and center selection for the LLRBFNN model, and the MFO algorithm was used to keep those centers up-to-date. We can observe, from our results, the high degrees of categorization accuracy. The suggested "MFO based LLRBFNN" model has improved classification accuracy, although it takes somewhat longer to compute than the "MFO-LLRBFNN" model. Features were extracted from the MR images using the GLCM feature extraction method. Better tumor reclassification outcomes were displayed by the proposed model. As an added bonus, the MFCM has been used to choose the centers of the LLWNNmodel and the MFO method was used to keep those centers up-to-date. When the proposed MFO-based LLWNN model was compared to the "MFO-LLWNN" model, it was discovered that the former required more "computational time," but the latter provided a more accurate classification. Using an optimized data set on a randomly generated forest classifier, a 99 % accuracy was achieved.
REFERENCES
1. Kv AM, Rajendran V et al. Glioma tumor grade identification using artificial intelligent techniques. J Med Syst. 2019 Mar 21;43(5):113. doi: 10.1007/s10916-019-1228-2.
2. Cui W, Wang Y, Fan Y, Feng Y, Lei T. Localized FCM clustering with spatial information for medical image segmentation and bias field estimation. International Journal of Biomedical Imaging. 2013;2013:930301. doi: 10.1155/2013/930301.
3. Wang G, Xu J, Dong Q, Pan Z. Active contour model coupling with higher order diffusion for medical image segmentation. International Journal of Biomedical Imaging. 2014;2014:237648. doi: 10.1155/2014/237648.
4. Chaddad A. Automated feature extraction in brain tumor by magnetic resonance imaging using Gaussian mixture models. International Journal of Biomedical Imaging. 2015;2015:868031. doi: 10.1155/2015/868031.
5. Mirjalili S, Seyedali M. Moth-flame optimization algorithm: a novel nature-inspired heuristic paradigm. Knowledge-Based Systems. 2015;89:228-249. doi: 10.1016/j.knosys.2015.07.006.
6. Charutha S, Jayashree MJ. An efficient brain tumor detection by integrating modified texture based region growing and cellular automata edge detection. 2014 Int. Conf. Control. Instrumentation, Commun. Comput. Technol. ICCICCT 2014. 2014;pp. 1193–1199. doi: 10.1109/ICCICCT.2014.6993142.
7. Iyyanar P, Arunachalam M, Patil AM, Uke N, Lal JD, Sonawane VR, Rajagopal R. A Real-Time 3D Video Streaming System Using SRTP and RTSP Protocol. IJCSNS. 2022;:45-54. doi: 10.22937/IJCSNS.2022.22.6.76.
8. Ashok K, Boddu R, Syed SA, Sonawane VR, Dabhade RG, Shaker Reddy PC. GAN base feedback analysis system for industrial IOT networks. Automatika. 2022;doi: 10.1080/00051144.2022.2140391.
9. Sonawane V, Rao DR. A comparative study: change detection and querying dynamic XML documents. International Journal of Electrical & Computer Engineering. 2015;5(4):840-848. doi: 10.11591/ijece.v5i4.pp840-848.
10. Sonawane V et al. A survey on mining cryptocurrencies. Recent Trends in Intensive Computing. 2021;:39-48. doi: 10.3233/APC210212.
11. Sonawane VR, Rao DR. An optimistic approach for clustering multi-version XML documents using compressed delta. International Journal of Electrical and Computer Engineering. 2015;5(6):1472-1479.
12. Kharade KG, et al. Text summarization of an article extracted from Wikipedia using NLTK library. In: Singh M, Tyagi V, Gupta PK, Flusser J, Ören T, Sonawane VR, editors. Advances in computing and data sciences. ICACDS 2021. Communications in Computer and Information Science. Springer; 2021. vol 1441. p. 375-385. doi:10.1007/978-3-030-88244-0_19.
13. Katkar SV, Kharade KG, Patil NS, Sonawane VR, Kharade SK, Kamat RK. Predictive modeling of tandem silicon solar cell for calculating efficiency. In: Singh M, Tyagi V, Gupta PK, Flusser J, Ören T, Sonawane VR, editors. Advances in computing and data sciences. ICACDS 2021. Communications in Computer and Information Science. Springer; 2021. vol 1441. p. 365-374. doi:10.1007/978-3-030-88244-0_18.
14. Kharade KG, Kharade SK, Sonawane VR, Bhamre SS, Katkar SV, Kamat RK. IoT based security alerts for the safety of industrial area. In: Recent trends in intensive computing. IOS Press; 2021. p. 98-103. doi:10.3233/APC210185.
15. Sonawane V, Rao DR. HCMX: an efficient hybrid clustering approach for multi-version XML documents. Journal of Theoretical and Applied Information Technology. 2015;82(1):137-148.
16. Sonawane VR, Singh LL, Nunse PR, Nalage SD. Visual monitoring system using simple network management protocol. In: 2015 International Conference on Computational Intelligence and Communication Networks (CICN). IEEE; 2015. p. 197-200. doi: 10.1109/ISITIA.2015.7220011.
17. Kumar P, Vijayakumar B. Brain tumour MR image segmentation and classification using PCA and RBF kernel-based support vector machine. Middle-East Journal of Scientific Research. 2015;23(9):2106–2116. Abdel M, Awadi RE. Brain tumor segmentation based on a hybrid clustering technique. 2015;71-81. doi: 10.5829/idosi.mejsr.2015.23.09.22458.
18. Despotović I, Philips W. MRI segmentation of the human brain: challenges, methods, and applications. 2015. doi:10.1155/2015/450341.
19. Chandra GR, Kolasani Ramchand H. Tumor detection in brain using genetic algorithm. Measurement. 2022;100412:449-457. doi:10.1016/j.measen.2022.100412.
20. Capelle C.A.S., Fernandez-Maloigne C. Evidential segmentation scheme of multi-echo M.R. images for the detection of brain tumors using neighbourhood information. Information Fusion. 2004;5:103-216. DOI: 10.1016/j.inffus.2003.10.001.
21. Ravi A, Sreejith S. A review on brain tumor detection using image segmentation. Int J Emerg Technol Adv Eng. 2015;5:60-64. DOI: 10.22214/ijraset.2021.39184.
22. Shen D, Wu G, Suk H.-I. Deep learning in medical image analysis. Annu Rev Biomed Eng. 2017;19:34-41. DOI: 10.1146/annurev-bioeng-071516-044442.
23. Huang M, Yang W, Wu Y, Jiang J, Chen W, Feng Q. Brain Tumor Segmentation Based on Local Independent Projection-Based Classification. IEEE Trans Biomed Eng. 2014 Oct;61(10):2633-2645. DOI: 10.1109/TBME.2014.2325410.
24. Clark M.C., Hall L.O., Goldgof D.B., Velthuizen R., Murtagh F.R., Silbiger M.S. Automatic tumor segmentation using knowledge-based techniques. IEEE Trans Med Imaging. 1998 Apr;17(2):187-201. DOI: 10.1016/j.jksuci.2018.11.001.
25. Bharanidharan N, Geetha V. Performance Analysis of KNN Classifier with and without GLCM Features in Brain Tumor Detection. 2018; pp. 103-106.
26. Seetha J, Selvakumar Raja S. Brain Tumor Classification Using Convolutional Neural Networks. 2018; pp. 1457-1461. DOI: 10.13005/bpj/1511.
Conflicts of interest
None.
Financing
None.
Authorship contribution
Conceptualization: Kumar Siddamallappa Ujjappanahalli, Vijay Ramnath Sonawane, Nisarg Gandhewar.
Methodology: Kumar Siddamallappa Ujjappanahalli, Vijay Ramnath Sonawane, Nisarg Gandhewar.
Writing - Original Draft: Kumar Siddamallappa Ujjappanahalli, Vijay Ramnath Sonawane, Nisarg Gandhewar.
Writing - Review & Editing: Kumar Siddamallappa Ujjappanahalli, Vijay Ramnath Sonawane, Nisarg Gandhewar.