SYSTEMATIC REVIEWS OR META-ANALYSES
Propofol infusion syndrome: a systematic review
Síndrome por infusión de propofol: una revisión sistemática
Rone Santos Lucas1 ![]() *, Marcelo Adrian Estrin1
*, Marcelo Adrian Estrin1 ![]() *
*
1Universidad Abierta Interamericana, Facultad de Medicina y Ciencias de la Salud, Ciudad Autónoma de Buenos Aires, Argentina.
Cite as: Santos Lucas R, Estrin MA. Propofol infusion syndrome: A systematic review. Salud Cienc. Tecnol. 2023;3:329. https://doi.org/10.56294/saludcyt2023329
Submitted: 23-01-2023 Revised: 14-02-2023 Accepted: 06-03-2023 Published: 09-03-2023
Editor: Dr.
William Castillo González ![]()
ABSTRACT
Introduction: propofol infusion syndrome (SIP) is a rare but extremely serious condition that can occur following the administration of high doses (>2 - 5 mg/kg/h) of propofol for prolonged periods (>48 hours). However, cases of SIP have also been reported after low-dose or short-duration propofol infusion. The condition is characterized by metabolic acidosis, cardiovascular and renal disturbances, rhabdomyolysis, as well as electrocardiographic abnormalities, etc.
Objective: to describe the main characteristics, prevention, diagnosis, and management of SIP.
Methods: a literature search and selection of articles published in the last 5 years on SIP in critically ill patients were carried out in English, Spanish, and Portuguese. This was done through the Pubmed, TRipDatabase, SciELO, and Google Scholar databases, following the PRISMA methodology.
Results: 26 articles were included, which analyzed the definition, general characteristics, epidemiology, risk factors, pathophysiology, clinical manifestations, prevention, and treatment.
Conclusions: the literature found reports that SIP is very rare but potentially fatal. The best treatment is prevention and early diagnosis. It is important to be aware that patients receiving propofol infusion are at risk of developing SIP. Therefore, greater monitoring and knowledge of patient clinical profiles is recommended. If SIP is suspected, propofol use should be discontinued and replaced with an alternative hypnotic agent, and management should be initiated. This includes immediate discontinuation of propofol infusion and problem-oriented management, such as hemodialysis, hemodynamic support, and extracorporeal membrane oxygenation in refractory cases. Further research is needed on SIP to obtain more data on its diagnosis, pathophysiology, and incidence.
Keywords: Propofol; Propofol Infusion Syndrome; Sedation; Critical State; Bradycardia.
RESUMEN
Introducción: el síndrome por infusión de propofol es un cuadro infrecuente pero extremadamente grave secundario a la administración de propofol a dosis elevadas (>2 - 5 mg/kg/h) y durante largos periodos (>48 hs). Pero también, se han informado casos de SIP después de la infusión de propofol en dosis bajas o de corta duración. Se caracteriza por la presencia de acidosis metabólica, alteraciones cardiovasculares y renales, rabdomiólisis, así como alteraciones electrocardiográficas, etc.
Objetivo: describir las características principales, prevención, diagnóstico y manejo del manejo del síndrome por infusión de propofol (SIP).
Métodos: se realizó una búsqueda bibliográfica y de selección de artículos de los últimos 5 años sobre el SIP en pacientes críticos tanto en idioma inglés, español y portugués. La misma se llevó a cabo a través de las bases de datos de Pubmed, TRipDatabase, SciELO y Google Académico, siguiendo la metodología PRISMA.
Resultados: se incluyeron 26 artículos, en los que se analizaron: la definición, características generales, epidemiologia, factores de riesgo, fisiopatología, manifestaciones clínicas, prevención y tratamiento.
Conclusiones: la literatura encontrada reporta el síndrome de infusión por propofol es muy poco habitual pero potencialmente mortal. El mejor tratamiento es la prevención y el diagnóstico temprano del mismo. Siendo consciente que existe la probabilidad de desarrollar SIP en pacientes que reciben infusión de propofol. Se recomienda un mayor seguimiento, y conocimiento de los perfiles clínicos de los pacientes. Frente a cualquier sospecha de SIP, se debe suspender el uso, reemplazar por un agente hipnótico alternativo, y llevar a cabo el manejo de este. El cual incluye, aparte de la interrupción inmediata de la infusión de propofol el manejo orientado al problema. Los mismos pueden ser hemodiálisis, soporte hemodinámico y oxigenación por membrana extracorpórea en casos refractarios. Se requiere nuevas investigaciones sobre SIP, para obtener más datos sobre su diagnóstico, fisiopatología e incidencia.
Palabras Clave: Propofol; Síndrome por Infusión de Propofol; Sedación; Estado Crítico; Bradicardia.
INTRODUCTION
Propofol infusion syndrome (SIP) is a rare but extremely serious condition that can occur following the administration of high doses (>4 - 5 mg/kg/h) of propofol, which is commonly used in intensive care units (ICUs)(1) for prolonged periods (>48 hours).(2,3) This syndrome is characterized by metabolic acidosis, lipidemia, hyperkalemia, rhabdomyolysis, changes in the electrocardiogram, and arrhythmias. It was first reported in pediatric patients and subsequently in adults in the early 1990s. Although the main risk factor for its development is prolonged administration of high doses of propofol, cases have been described in patients who received relatively low doses for short periods.(4)
Propofol is a hypnotic-sedative anesthetic widely used in various circumstances, due to its functionality, as it has a rapid onset of action and recovery from sedation.(5) It also produces multiple beneficial effects such as neuroprotection, anticonvulsant, and reduces cerebral metabolic demand, among others. During the COVID-19 pandemic, it was the first-choice drug for analgosedation in all patients who required mechanical ventilation due to severe acute respiratory failure.(6) However, it requires strict monitoring due to cases related to the presentation of SIP, which, if not diagnosed and treated early and timely, can lead to multiorgan dysfunction and death.(7)
Therefore, this systematic review aims to describe the main characteristics, prevention, diagnosis, and management of SIP.
METHODS
The systematic review was based on a literature search and article selection in the last 5 years on SIP in critically ill patients in English, Spanish, and Portuguese. This was done through the Pubmed, TRipDatabase, SciELO, and Google Scholar databases, following the PRISMA methodology.
The selected articles were considered of interest due to their comprehensive and up-to-date development on the subject under study. The inclusion criteria used were: original studies describing SIP, meta-analyses, systematic reviews, and case reports. Exclusion criteria were articles containing only an abstract, where correct translation is not possible, and studies based on animals.
RESULTS
A total of 26 articles were included, which analyzed the definition, general characteristics, epidemiology, risk factors, pathophysiology, clinical manifestations, prevention, and treatment of SIP. Of these 26 selected articles, 58 % corresponded to the period 2017 - 2022 and the remaining 42 % to the period 2001 - 2016. Furthermore, 54 % were literature reviews, 23 % were case studies, 8 % were case series studies, 11 % were systematic reviews with case series studies, and 4 % were literature reviews and case studies.
Definitions
There is a consensus among authors when defining propofol and its uses, as well as propofol infusion syndrome. The only exceptions are that articles specializing in the area of anesthesiology tend to develop the definition of propofol more than others.(1, 7, 8, 10, 11, 13, 21, 23) Regarding the definition of propofol infusion syndrome, some authors have documented the existence of SIP at low doses or for short durations in non-critically ill patients.(3, 14, 22)
• Definition of Propofol: a sedative-hypnotic agent that was introduced in 1977 and is now used in numerous procedures and has been extended to all medical and surgical specialties (table 1).(21)
|
Table 1. Current use of propofol |
|||
|
Induction of general anesthesia |
Induction and maintenance of the anesthesia generates |
Monitored anesthetic care |
Drug of choice for ambulatory surgery |
|
Initial indication of propofol which, combined with inhalation agents and opioids, constitutes part of "balanced anesthesia". |
In TIVA (Total Intravenous Anesthesia) techniques associated with opioids and also with muscle relaxants, if necessary. Because of its PK profile, propofol is the best qualified of all IV agents for maintenance of anesthesia. |
Procedures outside the operating room - Sedation in patients ventilated in ICU(1) Treatment of nausea and vomiting(2) |
It is the drug of choice for ambulatory surgery due to its rapid and complete anesthetic recovery and lower incidence of postoperative nausea and vomiting - Faster return of consciousness (awakening), with minimal residual effect on the CNS - Eye surgery - Electroconvulsive therapy - cardioversions. |
|
Source: pharmacokinetics of propofol infusion. Galiotti(21) |
|||
|
Note: 1. Propofol has been approved since 1993 for sedation of adult ICU patients, although it is not recommended for pediatric patients. 2. Propofol is indicated for the prevention and treatment of postoperative nausea and vomiting, as well as for patients undergoing chemotherapy. |
|||
• Definition of Propofol Infusion Syndrome: an infrequent but potentially lethal adverse reaction to the continuous intravenous infusion of propofol. There is a direct relationship between high doses (>4 mg/kg/h) and prolonged (>48 hours) administration.
General characteristics of propofol
Propofol is an excellent sedative-hypnotic drug (table 1) thanks to its pharmacokinetic and pharmacodynamic properties (table 2).(12) Like with the definition of propofol, the general characteristics of propofol are more extensively developed in articles specializing in the area of anesthesiology.
|
Table 2. Properties of propofol |
|
|
Pharmacodynamic Properties |
Pharmacokinetic Properties |
|
Rapid time to action (approximately 30 seconds) Decrease in blood pressure and heart rate with induction and maintenance of anesthesia. Ventilatory depression. |
Rapid distribution time (half-life 2 - 4 minutes). Rapid elimination (half-life 30 - 60 minutes). Extensive distribution. |
Epidemiology
The incidence of Propofol Infusion Syndrome (SIP) to date is unknown. However, authors agree that SIP is associated with high mortality rates. Hemphill et al.(3) analyzed 108 publications documenting 168 cases of SIP over the last 30 years (1988 - 2018). Similarly, Krajčová et al.(14) conducted a structured review of all cases published between 1986 and 2015, with a total of 153 patients. Another multicenter study carried out in 11 institutions in the United States by Russel and colleagues followed patients who received propofol infusion for more than 24 hours.(15) In 2010, Agudelo et al.(5) described their experience with prolonged continuous infusion of moderate doses of propofol in 71 of 222 critically ill pediatric patients.
Risk factors
The infusion rate and duration of propofol were the main risk factors for the development of SIP (table 3). There is literature that classifies SIP into primary risk factors, triggering factors, and genetic factors.(2,4,11)
|
Table 3. Risk factors |
|
|
Risk Factors |
Authors |
|
Prolonged high dose of propofol |
1,2,3,4,5,8,9,11,12,14,15,16,17,18,19,20,21,22,23,24,26 |
|
Use of corticosteroids |
2,3,4,5,8,11,12,15,19,20,21,24,26 |
|
Critical condition - Low carbohydrate supply |
2,3,4,11,15,19,21,22,24,26 |
|
Use of catecholamines - Neurocritical patients - Genetic factor - Mitochondrial disorder |
1,2,4,11,12,15,19,20,24,26 |
|
Genetic factor - Mitochondrial disorder |
2,3,4,11,12,15,19,21,24 |
|
Early age |
2,3,4,11,12,14,21,22,26 |
|
Association with vasopressors - Sepsis |
1,2,4,5,11,15,19,24 |
|
Upper respiratory infections |
2,4,11,15,19,20,24,26 |
|
Others |
2,4,11,15,19,20,24 |
|
Association with vasopressors |
5,8,18,20,26 |
|
Sepsis |
2,4,11,22,26 |
|
Upper respiratory infection |
2,4,5,11 |
|
Others |
21,17,26 |
Pathophysiology
The exact pathophysiology of SIP is still unknown. However, several hypotheses have been proposed, with the latest suggesting uncoupling of the mitochondrial electron transport chain and inhibition of fatty acid oxidation, leading to reduced ATP production.
Clinical manifestations
The clinical manifestations of SIP are variable and differ among authors (table 4). However, the most frequent characteristics according to the analyzed articles are metabolic acidosis, rhabdomyolysis, acute renal failure, and hyperkalemia.
|
Table 4. Clinical manifestations |
|
|
Clinical manifestations |
Authors |
|
Metabolic acidosis |
1,2,3,4,5,9,11,12,14,15,16,17,18,19,22,23,24,26 |
|
Rhabdomyolysis |
2,3,4,9,11,12,14,15,22,23,24,26 |
|
Acute renal failure |
2,4,9,11,12,15,18,19,20,23,26 |
|
Hyperkalemia |
2,3,4,11,12,15,18,19,20,26 |
|
Electrocardiogram changes |
3,4,12,14,15,16,20,24 |
|
Heart failure |
1,3,9,11,14,15,23,24 |
|
Arrhythmias |
1,4,9,11,12,20,22,26 |
|
Fever |
3,11,14,15,18,20,24 |
|
Lipidemia |
1,2,3,12,15,19 |
|
Hypertriglycemia |
4,9,11,15,17,19 |
|
Elevated lactate |
3,15,16,17,26 |
|
Elevated liver enzymes |
3,11,15,16,26 |
|
Hypotension |
3,11,14,24 |
|
Hemodynamically unstable |
4,19,20,22 |
|
Myocytosis |
2,4,11,26 |
|
Hepatomegaly |
2,3,26 |
|
Hepatic failure |
2,5,18 |
|
Myocytosis |
2,11,26 |
Prevention
Figure 1 below shows the preventive measures reported in the literature.
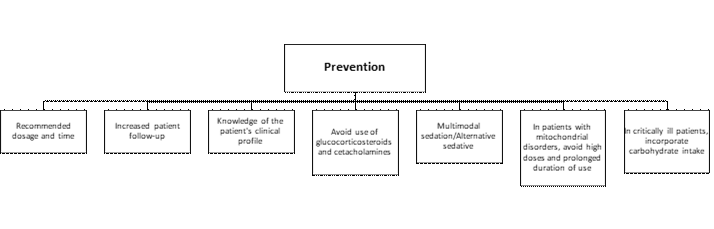
Figure 1. Prevention of SIP
Diagnosis and Treatment
There is no specific diagnosis for SIP, but authors agree that early diagnosis through increased monitoring of patients receiving propofol infusion is the best preventive tool available at present. Regarding treatment, there is currently no specific protocol. Consequently, treatment is supportive.
DISCUSSION
Propofol is a widely used intravenous anesthetic agent that was first introduced in 1977 and is still used in numerous procedures today due to its pharmacodynamic and pharmacokinetic properties. Its anesthetic properties were first described in 1973, which primarily involve an increase in inhibitory tone mediated by gamma-aminobutyric acid (GABA) at GABA-A receptors, leading to an increase in chloride influx into postsynaptic neurons, inhibiting the transmission of electrical impulses and causing varying levels of sedation.(8) In 1986, it was approved in Europe as an agent for the induction and maintenance of anesthesia in adults and children over 3 years of age, as well as for sedation and support of mechanical ventilation in the Intensive Care Unit (ICU).(9) It belongs to the group of alkylphenols, which are oils at room temperature and insoluble in aqueous solutions.(7) Its formulation has changed over the years, and currently, it is presented in a lipid emulsion form with a pH of 7, slightly viscous with a milky white appearance, and stable at room temperature.
It should be noted that commercial propofol emulsions, due to their pharmaceutical composition, lack antimicrobial preservatives. Therefore, in case of contamination, growth of various microorganisms can occur. The administration of microbiologically contaminated propofol, after being removed from its original container, may be associated with outbreaks of postoperative infection.(13) The expiration date of propofol emulsions is two years from the date of manufacture.
This anesthetic is associated with propofol infusion syndrome (SIP), a rare but highly lethal complication.(3) This syndrome was first described in 1990 after the death of a 2-year-old pediatric patient following prolonged and high-dose infusion of this drug. Subsequently, reports were published in adults. In 1998, Bray recognized SIP as a diagnostic entity through a compilation of cases in the pediatric population in Intensive Care Units (ICU), characterizing it as a set of symptoms: marked bradycardia of sudden onset resistant to treatment that can progress to asystole as a mandatory finding, plus the presence of at least one of the following: lipemic serum, clinical hepatomegaly, or evidence of fatty infiltration of the liver, metabolic acidosis with at least one arterial blood gas measurement showing a base deficit of ≥10 mmol/l, and rhabdomyolysis or myoglobinuria, in relation to patients who have been exposed to high doses of propofol (>4 - 5 mg/kg/h or >83 µg/kg/min) for long periods (>48 h).
In response, the US Food and Drug Administration (FDA) issued a warning against the use of propofol in continuous infusions, which was updated in 2006 to inform a maximum dose of 4 mg/kg/h. Similarly, the European Medicines Agency (EMA) recommends active monitoring of signs suggestive of SIP in patients with prolonged propofol infusions.(10)
Although SIP is a rare pathology that is difficult to identify in the ICU, it is potentially lethal and is associated with the use of high-dose and long-term propofol infusions.(12) However, there are reported cases of propofol infusion syndrome that have occurred at low cumulative doses.(3) For example, a critically ill patient was administered propofol for 20 days at an average dose of 1,3 mg/kg/h due to agitation and ventilation synchronization problems, with a successful outcome.(22) Similarly, in the analysis conducted by Krajcová et al.(14) of all case reports published until 2014, the latest cases corresponded to older patients who received infusions within the recommended dose limits.
Regarding epidemiology, the true incidence of SIP is unknown. This is because the available data are from isolated case reports or case series, and there are currently no unified criteria for its diagnosis, associated pathologies, or risk factors. Russell et al.(15) conducted a multicenter prospective study in 1,017 adults, collecting nine clinical manifestations related to this syndrome. Only 1 % of the cases presented propofol infusion syndrome when receiving this drug for more than 24 hours, with a mortality rate of 18 %. SIP also appeared at low doses of propofol. Most patients survived. Reports of mortality attributable to SIP range from 30 % - 80 %.
Another study by Cremer et al. reported an incidence of 10 % in neurocritical adults treated with propofol, but this increased to 31 % in patients who received doses greater than 6 mg/kg/h.(4) Hemphill et al.(3), after analyzing 108 publications documenting 168 cases of SIP in the last 30 years (1988 - 2018), concluded that the mortality rate for SIP was 48 % in adults and 52 % in pediatric patients. Similarly, Krajčová et al.(14) conducted a structured review of all cases published between 1986 and 2015, totaling 153 patients (1990 - 2014), of whom 78 (51 %) had a fatal outcome. In 138 cases (90 %), patients developed SIP as a complication of non-procedural sedation in the ICU, and in 15 cases (10 %), as a complication of propofol use during anesthesia. The reported mortality rates in the reported cases decreased over time from 74 % (20 - 27 %) before 2001 to 64 % (34 - 53 %) between 2001 and 2006, and to 32 % (23 - 72 %) in cases reported after 2006.
Agudelo et al.(5) described their experience with prolonged continuous infusion of moderate doses of propofol in 71 out of 222 critically ill pediatric patients (32 %). The mean propofol dose was 2,1 mg/kg/h and the mean duration was 6,7 days. The mean age was 45,8 months. No patient developed propofol infusion syndrome or any other serious adverse effects. The latter author maintains that in their experience of more than 15 years of continuous propofol infusion in over 1,000 treated patients, they have never observed this syndrome.
In 2016, the first case of a patient developing propofol infusion syndrome after an emergency cesarean delivery of a premature baby was documented (the first case in the obstetric population).(16)
Regarding risk factors, the authors agree that the greatest factor is high and prolonged infusion dose of propofol, accounting for 26 %. Accumulation of propofol has also been linked to the syndrome.(3, 9, 12, 20) Although cases have been reported in critical care units with low infusion rates.(20) This is followed by corticosteroid use, critical illness, and low carbohydrate intake, accounting for 16 % and 13 %, respectively.
Propofol infusion syndrome has been observed in patients with mitochondrial disease (10 %), so it is possible that corticosteroid administration acts as a predisposing factor for the development of propofol infusion syndrome.(3) On the other hand, an increase in catecholamines, accounting for 11 %, leads to increased clearance of propofol, which can potentially require a higher dose of propofol.(19)
Critical illness (13 %) is involved in the development of propofol infusion syndrome, while traumatic brain injury has been linked to death from the syndrome.(15) According to the analyzed literature, the neurocritical state accounts for 11 %.
Other potential risk factors for the development of propofol infusion syndrome include young age at 9 %, association with vasopressors and sepsis at 6 %, upper respiratory infections at 5 %, and other factors representing 4 %.
Regarding the latter, it includes obesity,(20) carnitine deficiency,(26) high dose of methylprednisolone during induction,(17) and the elderly.(11) Pardo et al. consider obesity a risk factor.(20) In contrast, in the study conducted by Hemphill et al.(3), they could not identify any relationship between patient weight or BMI and the development of propofol infusion syndrome or mortality from it.
Other authors classify factors(2,4,11) as primary risk factors (mostly developed from critical illness and resulting in the production of endogenous catecholamines, glucocorticoids, and endogenous cytokines, contributing to cardiac, peripheral, and hepatic muscle dysfunction); Trigger factors (high doses of propofol (>4 mg/kg/h), prolonged periods (>48 h), and infusion of catecholamines and corticosteroids); and finally, genetic factors (acyl coenzyme A dehydrogenase efficiency, low carbohydrate intake, young age, previous defect in beta-oxidation, and pre-existing mitochondrial disorders).
Galiotti(21) maintains that there are patients at greater risk for developing propofol infusion syndrome: patients with severe brain trauma receiving propofol infusions at doses ≥ 5 mg/kg/h have twice the risk of developing the syndrome, and in patients with severe burns, trauma, sepsis, pancreatitis or status asthmaticus, high doses and prolonged use of propofol should be avoided.
It is important to consider that some of these risk factors may favor the development of propofol infusion syndrome,(19) as it is not known with certainty whether these factors represent only a marker of some serious illness or if they play a relevant role in the face of SIP.
Regarding the pathophysiology, it is still not fully elucidated, and it is complex and multifactorial.(17) It is mainly characterized by cellular injury and death secondary to the imbalance between intracellular energy supply and demand.(20)
Clinical and experimental studies have found a relationship between the use of propofol and a disturbance in the use of free fatty acids in mitochondrial activity and an imbalance between energy demand and utilization during states of nutritional deficiency.(2,18) In other words, mitochondrial electron transport chain uncoupling and inhibition of fatty acid oxidation occur,(11) resulting in the accumulation of fatty acids in the mitochondria. This results in respiratory chain dysfunction with reduced ATP production.(3,18)
The energy deficit particularly affects skeletal and cardiac muscle cells, causing cell lysis and the development of rhabdomyolysis and myocardial dysfunction, respectively.(4,17,18,19)
Regarding clinical manifestations, they are variable. The most commonly reported by the analyzed authors are metabolic acidosis (21 %), rhabdomyolysis (14 %), acute renal failure (13 %), hyperkalemia (12 %), and changes in electrocardiogram (10 %). This is because it is difficult to recognize SIP symptoms in the early stages because they are nonspecific and overlap with signs commonly seen in critically ill patients with shock and organ dysfunction. Furthermore, some of the symptoms reflect common pharmacological manifestations of propofol, such as bradycardia.(22) Metabolic acidosis and rhabdomyolysis usually occur early in SIP, but these symptoms may be masked in a patient receiving continuous renal replacement therapy (CRRT) for acute renal failure, delaying the diagnosis of SIP.(3)
On the other hand, Hemphill(3) and Pardo(20) state that the most frequent characteristic is metabolic acidosis, which affects almost 80 % of children and adults, followed by changes in electrocardiogram, which is the second most common characteristic (75 % of children and around 63 % of adults). Additionally, Avila et al.(2), Carassi et al.(11), and Muzaiwirin(23) agree that 80 % of clinical manifestations consist of metabolic acidosis, without specifying the age range, followed by rhabdomyolysis, which can be present in 40 - 60 % depending on the cardiovascular pathology. Other reported manifestations include hypertriglyceridemia (20 - 40 %), elevated liver enzymes (10 – 20 %), and fever (10 – 40 %).
The most frequently involved systems are the cardiovascular, hepatic, skeletal, renal, and metabolic systems (Table III).(2, 11, 23) In contrast, Hemphill et al.(3) classified them into cardiac disorders, vascular disorders, renal and urinary disorders, musculoskeletal and connective tissue disorders, metabolism and nutrition disorders, and hepatobiliary disorders.
To conclude this topic, clinical manifestations of SIP are variable. The most common ones among the analyzed authors are metabolic acidosis (21 %), rhabdomyolysis (14 %), acute renal failure (13 %), hyperkalemia (12 %), and electrocardiogram changes (10 %). These symptoms are difficult to recognize in the early stages because they overlap with common signs in critically ill patients with shock and organ dysfunction, and other pharmacological manifestations of propofol, such as bradycardia.(22) Metabolic acidosis and rhabdomyolysis usually occur in the early stage of SIP, but these symptoms may be masked in a patient receiving continuous renal replacement therapy for ARF, delaying the diagnosis of SIP.(3)
In terms of prevention, early diagnosis is the best approach.(3) Knowledge of clinical profiles and increased monitoring of patients receiving propofol infusion are the best measures for early diagnosis.(11, 17, 19) Administering the medication under the correct indications, according to recommended dose and time.(2) Limiting the infusion to doses of 4 - 5 mg/kg/h for no more than 48 hours.(2, 3, 11, 19) Also, strict monitoring and control of physiological variables and laboratory parameters, as they may indicate the early development of SIP.(2, 9, 11, 19)
Whenever feasible, glucocorticosteroids and catecholamines should be avoided for patients receiving propofol.(25) If propofol sedation will be prolonged or a high dose cannot be avoided, such as ECMO application, shock, and neurological injuries, physicians should use propofol at the smallest dose possible, using a multimodal sedation regimen and switching to alternative sedatives if the patient receives propofol at high doses (>5 mg/kg/hr) or prolonged periods (>48 hours) or at high accumulated doses (>240 mg/kg).(21, 22)
Patients with mitochondrial disorders should not receive high doses or prolonged periods of propofol as a preventive measure.(2) Propofol has shown alterations in the mitochondrial respiratory chain, so patients with mitochondrial disorders should not receive high doses or prolonged periods of propofol as a preventive measure.(18, 24) It is important to know sedation guidelines in the management of these patients.(20)
Pediatric and adult critically ill patients should maintain an intake of 6 - 8 mg/kg/min of carbohydrates per day to prevent the onset of SIP.(2, 24) This is because carbohydrate reserves deplete faster, so inadequate intake of these nutrients promotes mobilization of fat deposits (increasing their metabolism) and circulating free fatty acid load, which predisposes to SIP.(2, 17)
To date, there is no specific diagnostic test for Propofol Infusion Syndrome (SIP). Early diagnosis is the most valuable tool available to achieve successful treatment. Diagnosis consists of continuous monitoring of clinical and laboratory tests for physiopathological alterations, and follow-up with lactate acid and CPK while the patient is receiving Propofol infusion. (CPK>5000 I UL, Lactic Acid>4).(2, 23) It is also important to be aware that there is a possibility of developing SIP if the patient is receiving the drug and presents any clinical manifestations.
Continuous monitoring of clinical and laboratory tests for physiopathological alterations and the patient's clinical condition is recommended.(2) For cardiovascular clinical manifestations, electrocardiogram; for musculoskeletal manifestations, myoglobinuria, creatine phosphokinase, creatinine, complete electrolytes, for metabolic manifestations, arterial gases, lactic acid, lipid profile; and for hepatic manifestations, bilirubin, transaminases, alkaline phosphatase, and coagulation times.(2, 11, 23)
However, metabolic acidosis and heart failure occur early and in a dose-dependent manner, while rhabdomyolysis, arrhythmias, and other changes in the electrocardiogram are more dependent on the duration of Propofol infusion.(2, 26)
Treatment for SIP is directed at the clinical manifestation because there are no established treatment guidelines or antidote for this syndrome.(20) It includes the immediate suspension of Propofol infusion, maintaining sedation using an alternative hypnotic agent such as dexmedetomidine or midazolam,(3) as well as general supportive measures and cardiopulmonary and renal support if necessary.(11, 17)
The success of treatment depends on a high index of suspicion as well as early diagnosis.(3) As mentioned above, there is no guide available to direct SIP treatment. Prevention can also be considered the best treatment.(2)
Regarding severe cases, studies have shown that hemodialysis and hemodiafiltration, if required to decrease lipid and acid compound concentrations, are very effective for managing cases of SIP. However, these interventions have been ineffective in the treatment of fatal cases.(2,7) In contrast, Pardo-Ruiz and colleagues argue that hemodialysis or hemofiltration are useful techniques that allow for the elimination of propofol along with its toxic metabolites.(4,20)
A recent study by Parque et al. used L-carnitine as a treatment for SIP. They considered that L-carnitine supplementation was unlikely to cause harm and could be beneficial given the etiology of SIP. The patient showed rapid recovery after L-carnitine supplementation.(20) Although the authors are not sure of L-carnitine's role, they recommend more studies on this topic, given the favorable results they obtained.
CONCLUSIONS
Propofol infusion syndrome is very rare but potentially deadly. It mainly occurs due to the use of high-dose (greater than 4 mg/kg/h) and prolonged (>48 hours) propofol infusions. However, cases have also been reported where SIP has been triggered by low doses or short durations in non-critically ill patients. Therefore, prevention and early diagnosis are the best treatment options. It is important to be aware that patients receiving propofol infusion are at risk of developing SIP, and to monitor their clinical profiles closely.
If SIP is suspected, the use of propofol should be stopped, replaced with an alternative hypnotic agent, and managed accordingly. Management may include immediate interruption of propofol infusion, hemodialysis, hemodynamic support, and extracorporeal membrane oxygenation in refractory cases.(26) Alternatives to long-term sedation should also be considered. If this is not possible, strict patient monitoring is essential.
Further research is needed to obtain more data on the diagnosis, pathophysiology, and incidence of SIP.
REFERENCES
1. Fudickar A, Bein B, Tonner PH. Propofol infusion syndrome in anaesthesia and intensive care medicine. Cur Opi Ana. 2006; 19 (4): 404-10. Disponible en: https://pubmed.ncbi.nlm.nih.gov/16829722/
2. Ávila L, Tabares M, Londoño M. Síndrome de infusión por propofol: revisión de tema. Uni Med. 2021; 62 (2).
3. Hemphill S, McMenamin L, Bellamy M, Hopkins P. Propofol infusion syndrome: a structured literature review and analysis of published case reports. Brit de Ane. 2019; 122 (4): 448-459.
4. Martinez V, Angulo M, Barbato M. Síndrome por infusión de propofol: reporte de un caso. Rev Méd Urug. 2017; 33 (3): 211-213.
5. Agudelo M, Mencia S, Faro A, Escudero V, Sanavia E, Lopez-Herce E. Propofol en perfusión continua en niños en estado crítico. Med Int. 2012; 36 (6): 410-415.
6. Carini F, Cassabella C, Garcia M. Analgosedación en el paciente crítico en ventilación mecánica: el bundle ABCDEF en la pandemia de COVID-19. Rev Arg de Ter Int. 2020; 1: 47-53.
7. Muñoz-Cuevas J, Cruz-Paz M, Olivero-Vásquez Y. Propofol ayer y hoy. Rev Mex de Ane. 2005; 28:148-158
8. Timbó B. Propofol infusion syndrome. Rev Bras Anestesiol. 2007; 57: 539-542.
9. Chidambaran V, Costandi A, D'Mello A. Propofol: A review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015; 29(7): 543-63.
10. Chollet-Riviera M, Chiole A. Anaesthesia for procedures in the intensive care unit. Cur Opi in Ana. 2001; 14: 447-45.
11. Caracci B, Aranda F. Síndrome de infusión por propofol en el adulto. Rev chi de ane. 2018; 47 (3): 189-195.
12. Carrillo-Esper R, Garnica M, Bautista-León R. Sindrome de infusión por propofol (SIP). Rev Mex de Ane. 2010; 33 (2): 97-102.
13. Amabile-Cuevas C, Lepe-Mancilla J, Muñoz-Cuevas J. El efecto inhibitorio del EDTA sobre el crecimiento microbiano en suspensiones de propofol. Rev Mex de Ane. 2019; 42(2): 104-110.
14. Krajčová A, Waldauf P, Michal A, František D. Síndrome de infusión de propofol: una revisión estructurada de estudios experimentales y 153 informes de casos publicados. Rev Cri Car. 2015; 19 (398).
15. Russel R, Jeffrey B, Jeffrey F, Greg S, Kuper F, Papadopoulos S,Yogaratnam D, Kendall E , Xamplas R , Gerlach A, Szumita P , Eira K , Arpino P , Voils S , Grgurich F, Ruthazer R , Devlin J. Rev Cri Car. 2019; 13 (5).
16. Akwugo E, Solafa E, Mili T, Rocha F. Síndrome de infusión relacionado con propofol en el período periparto. 2016 ;6(4):368-371. https://doi.org/10.1055/s-0036-1593405.
17. Dehesa-Lopez E, Irizar-Santana S, Granado R, Ortiz R. Síndrome de infusión de Propofol en el postoperario de un trasplante renal. 2019; 2019: 7498373. https://doi.org/10.1155/2019/7498373.
18. Vollmer J, Haen S, Wolburg H, Lehmann R, Steiner J, Reddersen S. Síndrome de infusión relacionado con propofol: evidencia ultraestructural de un trastorno mitocondrial. Cri Car Med. 2018; 461: 91-94. https://doi.org/10.1097/ccm.0000000000002802.
19. Vasile B, Rasulo F, Candiani A, Latronico N. La fisiopatología del síndrome de infusión de propofol: un nombre simple para un síndrome complejo. Rev Med Cui Int. 2003; 29 (9): 1417–1425.
20. Pardo C, Ruiz- Hernández G, Jiménez- Orduz A, Archila Tibaduiza. Síndrome por Infusión de Propofol, una perspectiva en las Unidades de Cuidado Intensivo. Universidad de Santander (UDES), Colombia Anestesióloga Hospital Internacional de Colombia HIC. 2021.
21. Galiotti G. Farmacocinética del propofol en infusión. Rev Ane Arg. 2019; 67 (2): 154-185.
22. Parque N, Sun Ha T. Tratamiento exitoso del síndrome de infusión relacionado con propofol en pacientes en estado crítico que reciben una infusión de propofol en dosis bajas. Acute and Critical Care 2021;38(1):144-148. https://doi.org/10.4266/acc.2021.00829.
23. Muzaiwirin M, Utariani A. Protocolo de cribado del síndrome de infusión de propofol. Ind Jou of Ane and Rea. 2020: 2 (2). https://doi.org/10.20473/ijar.V2I22020.67-76.
24. Baltodamo-Torres B. Síndrome por infusión de propofol en la población general. Rev Ele de Por Med. 2021; 16 (21): 990.
25. Lu L, Weixi X, Yingying Z, Yingfeng X, Dong Z. Estado epiléptico refractario inducido por propofol en edad de remisión en epilepsia benigna con puntas centrotemporales: reporte de un caso y revisión de la literatura. 2019; 98(27): 16257. https://doi.org/10.1097/md.0000000000016257.
26. Mirrakhimov AE, Voore P, Halytskyy O, Khan M, Ali AM. Propofol Infusion Syndrome in Adults: A Clinical Update. Critical Care Research and Practice 2015;2015:e260385. https://doi.org/10.1155/2015/260385.
FUNDING
No financing.
CONFLICTS OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Conceptualization: Rone Santos Lucas, Marcelo Adrian Estrin.
Investigation: Rone Santos Lucas, Marcelo Adrian Estrin.
Methodology: Rone Santos Lucas, Marcelo Adrian Estrin.
Writing - original draft: Rone Santos Lucas, Marcelo Adrian Estrin.
Writing - review and editing: Rone Santos Lucas, Marcelo Adrian Estrin.