REVIEW
An emerging Microbe for Food Enzyme Production in Biomanufacturing
Un microbio emergente para la producción de enzimas alimentarias en la biofabricación
Renuka Jyothi Shettru1
![]() *,
Divya Shrivastava2
*,
Divya Shrivastava2 ![]() , Sudhir
Singh3
, Sudhir
Singh3 ![]()
1Department of Life Science, School of Sciences, JAIN (Deemed-to-be University). Bangalore, India.
2School of Life and Basic Sciences, Jaipur National University. Jaipur, India.
3Department of Microbiology, Teerthanker Mahaveer University. Moradabad, Uttar Pradesh, India.
Cite as: Jyothi Shettru R, Shrivastava D, Singh S. An emerging Microbe for Food Enzyme Production in Biomanufacturing. Salud, Ciencia y Tecnología. 2023;3:410. https://doi.org/10.56294/saludcyt2023410
Received: 23-04-2023 Reviewed: 11-05-2023 Accepted: 09-06-2023 Published: 10-06-2023
ABSTRACT
This article provides an overview of the characteristics, applications, and potential of Aspergillus niger in dietary enzyme production. A. niger is a filamentous fungus that grows naturally in a wide variety of temperatures and pH levels. It is frequently utilized in the synthesis of citric acid and other organic acids. A. niger is also a significant organism in traditional fermented foods, and it has been utilized to make food enzymes that have been designated as Generally Recognized As Safe (GRAS) by the United States Food and Drug Administration. The constraints of A. niger genetic manipulation are discussed, as well as the need to eradicate mycotoxins in industrial strains. The article finishes with an overview of current advances in genetic manipulation and enzyme overproduction tactics, which may assist to increase the efficiency and safety of A. niger as a potential enzyme host in the food industry.
Keywords: A. Niger; Food Enzyme Production; Biomanufacturing; Drug Administration.
RESUMEN
Este artículo ofrece una visión general de las características, aplicaciones y potencial de Aspergillus niger en la producción de enzimas alimentarias. A. niger es un hongo filamentoso que crece de forma natural en una amplia variedad de temperaturas y niveles de pH. Se utiliza con frecuencia en la síntesis de ácido cítrico y otros ácidos orgánicos. A. niger es también un organismo importante en los alimentos fermentados tradicionales, y se ha utilizado para fabricar enzimas alimentarias que han sido designadas como Generally Recognized As Safe (GRAS) por la Administración de Alimentos y Medicamentos de Estados Unidos. Se discuten las limitaciones de la manipulación genética de A. niger, así como la necesidad de erradicar las micotoxinas en las cepas industriales. El artículo concluye con una panorámica de los avances actuales en manipulación genética y tácticas de sobreproducción enzimática, que pueden contribuir a aumentar la eficacia y seguridad de A. niger como potencial huésped enzimático en la industria alimentaria.
Palabras clave: A. Niger; Producción de Enzimas Alimentarias; Biofabricación; Administración de Drogas.
INTRODUCTION
In recent years, there has been a surge of interest in the use of microorganisms in biomanufacturing for the synthesis of food enzymes. A. oryzae, a filamentous fungus that has long been utilized for food fermentation in Asia, is one such developing microorganism.(1) Due to its ability to secrete a diverse spectrum of enzymes, including amylases, proteases, and lipases. Furthermore, developments in genetic engineering have allowed A. oryzae to be optimized for improved enzyme synthesis, making it a good option for the biomanufacturing of food enzymes. As research into the potential of this bacterium proceeds, A. oryzae is likely to play an increasingly vital role in the food business and beyond. A. oryzae is a new microorganism that is garnering interest for its potential in the synthesis of food enzymes for biomanufacturing.(2,3) A. oryzae is a filamentous fungus with a wide range of enzyme-secreting activities that have traditionally been employed in Asian food fermentation. Recent genetic engineering developments have allowed A. oryzae to be optimized for improved enzyme production, making it an attractive candidate for industrial uses. Its non-toxic and non-pathogenic nature, as well as its ability to grow on a wide range of substrates, makes it an appealing alternative for long-term enzyme production. Food, biofuel, pharmaceutical, and cosmetic industries could all benefit from A. oryzae.(4)
A. oryzae is a filamentous fungus that has attracted attention for its potential in the production of food enzymes and biomanufacturing. A. oryzae has a long history of usage in traditional Asian food fermentation processes such as sake, soy sauce, and miso manufacture. It is a viable contender for commercial enzyme production due to its capacity to secrete a wide spectrum of enzymes such as amylases, proteases, and lipases. In recent years, researchers have concentrated on genetic engineering and fermentation process modification to optimize A. oryzae for increased enzyme production. As a result, A. oryzae strains capable of producing large quantities of certain enzymes such as alpha-amylase, glucoamylase, and protease have emerged. These enzymes have several uses in the food sector, including the manufacturing of sweeteners, starch-based products, and fermented meals.(5) A. oryzae enzymes have potential use outside of the food business, including biofuel production, medicines, and cosmetics. Furthermore, A. oryzae has various advantages over traditional enzyme production methods, such as reduced costs, higher yields, and greater control over the manufacturing process. Because A. oryzae is non-toxic and non-pathogenic, it provides a safe and sustainable option for enzyme synthesis. With continuous research and development, A. oryzae is projected to play an important role in the biomanufacturing of food enzymes and other industrial uses.(6) This article presents an overview of the properties, applications, and prospects of Aspergillus niger in the synthesis of dietary enzymes.
DEVELOPMENT
Manipulation of genes
Genetic manipulation of A. niger strains is far more difficult, time-consuming, and unsuccessful when compared to that of Escherichia coli and Bacillus subtilis. As a consequence of this, the development of effective tools for the modification of molecules is a vital prerequisite for boosting the amount of protein produced.
Transformation in A. niger
Aspergillus niger, a fungus frequently employed in the food business, may be genetically modified to increase the production of food enzymes. Researchers can increase the production of particular enzymes by altering the genetic makeup of the fungus, which can boost the efficacy and economy of food production operations.(7)
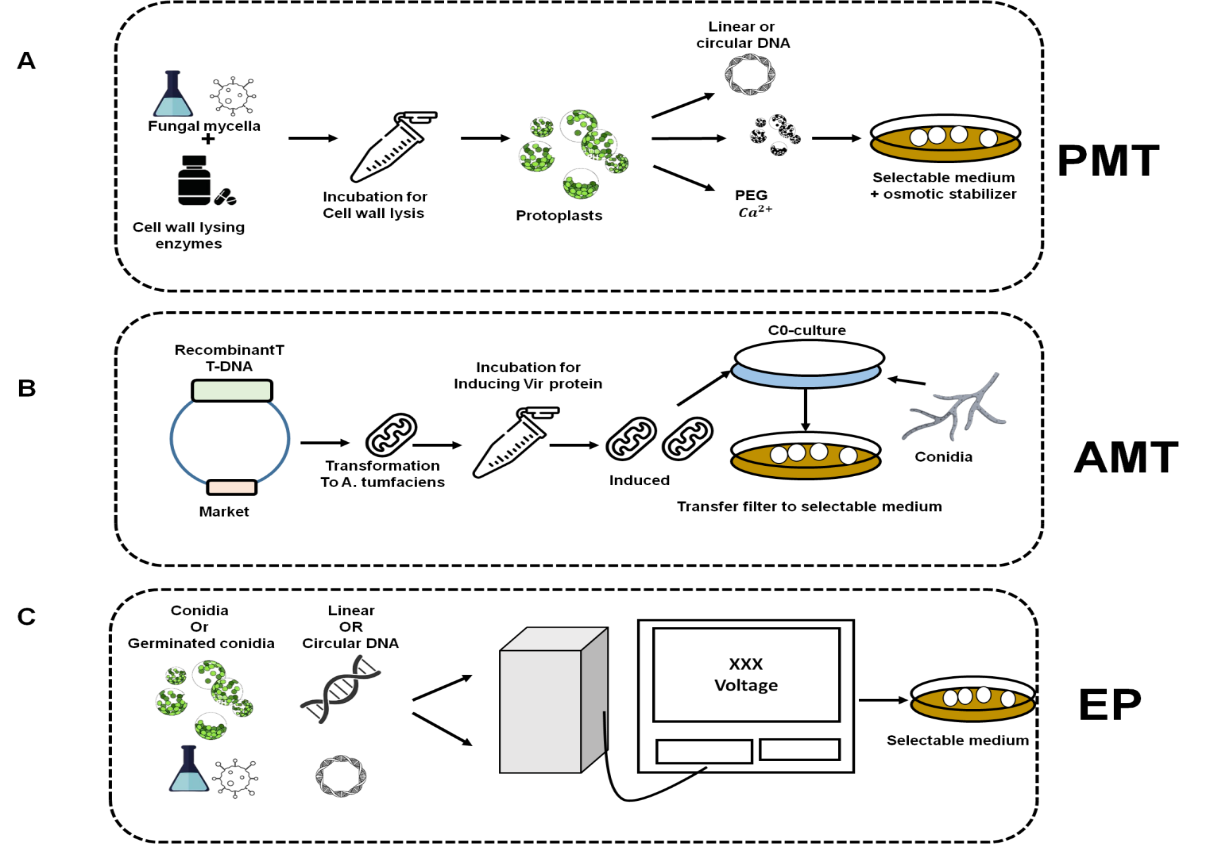
Figure 1. Techniques for transformation created for A. niger. Gray arrows depict the A. niger life cycle
PEG-mediated transformation
Aspergillus niger, a fungus frequently used in the production of food enzymes, is one organism that can have foreign DNA inserted into its genome using the genetic engineering technique known as PEG-mediated transformation.(7) Using polyethylene glycol (PEG), the fungal cell walls are made permeable in this technique, allowing foreign DNA to enter the cells and ingest into the genome. The organism can be modified to produce particular enzymes with enhanced properties for food production once the foreign DNA has been incorporated. Enzymes like glucoamylase, alpha-amylase, and pectinase, which are used in the food industry to make beer, wine, and fruit juice, respectively, have all been produced using this method.(8) The development of more effective and economical enzyme production processes has been made possible by PEG-mediated transformation, which has proven to be a useful tool in the production of food enzymes.(7) A. niger strains produce excellent enzymes via both solid-state and submerged fermentation table 1.
|
Table 1. Proteins synthesized by A. niger host strains |
||||
|
Protein |
Yield |
Gene source |
Fermentation method |
Host |
|
Glucanase |
4,523 g/L |
- |
SMF in shake flasks for 96h |
A. niger US368 |
|
Asparaginase |
5,54 g/L |
- |
SMF in 5L bioreactor for 96h |
A. niger AKV-MKBU |
|
Naringinase |
45 mg/g |
- |
SSF in shake flasks for 96h |
A. niger MTCC 1344 |
|
Glucoamylase |
13,78 g/L |
- |
SMF in shake flasks for 168h |
A. niger NRRL 330 |
|
Pectinase |
7,24 g/L |
- |
SMF in shake flasks for 168h |
A. niger |
|
Phospholipase A2 |
10 mg/ L |
Pig |
SMF in shake flasks for 168h |
A. niger AB4.1 |
|
Lysozyme |
70 mg/ L |
Hen egg |
SMF in shake flasks for 168h |
A. niger AB4.1 |
|
Interleukin-6 |
15 mg/ L |
Human |
SMF in shake flasks for 168h |
A. niger AB1.13 |
|
Antigenic proteins (nucleoside hydrolase and sterol 24-cmethyltransferase) |
54 mg/ L |
Leishmania sp. |
SMF in shake flasks for 168h |
A. niger ATCC 1015 |
|
Lipase |
126,8 mg/g |
- |
Solid State Fermentation (SSF) in shake flasks for 72h |
A. niger NCIM 1207 |
|
Lipase |
8,25 g/L |
- |
Solid State Fermentation (SMF) in shake flasks for 168h |
A. niger UV_ 10 |
|
Prolyl endopeptidase |
10,9 mg/g |
- |
SSF shake in flasks for 168h |
A. niger ATCC 11414 |
|
Mannanase |
1,23 g/L |
Phanerochaete chrysosporium |
SMF in shake flasks for 120h |
A. niger N593 |
|
Mannanase |
28,91 g/L |
Aspergillus. aculeatus |
SMF in shake flasks for 168h |
A. niger D15 |
|
Chymosin |
0,9 g/L |
Aspergillus. aculeatus |
SMF in shake flasks for 168h |
A. niger BM27 |
|
Preoxidase |
100 mg/L |
P. chrysosporium |
SMF in shake flasks for 168h |
A. niger NCIM MGG029 |
|
Xylanase |
3,11 mg/g |
- |
SSF in shake flasks for 96h |
A. niger USM SD2 |
|
Xylanase |
1,87 g/L |
- |
SMF in shake flasks for 168h |
A. niger US368 |
Transformation was mediated by the bacteria Agrobacterium tumefaciens
Transformation with Agrobacterium tumefaciens is a common method for introducing exogenous DNA into fungal cells. The method makes use of a binary vector system that is made up of a Ti plasmid that is produced from A. tumefaciens as well as a binary vector that contains the gene that is wanted. The T-DNA region of the Ti plasmid is responsible for the transfer of the gene of interest to the fungal genome.(7) The binary vector is introduced into A. tumefaciens, which is then co-cultured with cells from A. niger. During co-cultivation, A. tumefaciens cells transfer the Ti plasmid's T-DNA region to A. niger cells, where it can integrate into the fungal genome. The transformed fungal cells can be identified using antibiotic markers found on the binary vector. Transformation by Agrobacterium tumefaciens is a powerful tool for genetic engineering in fungi that has been successfully applied to several species, including A. niger.(9)
Electroporation
Electroporation is a common method for introducing foreign DNA into cells. This method involves exposing cells to a brief, high-voltage electrical pulse, which temporarily increases cell membrane permeability and allows foreign DNA to enter the cell. Electroporation is an effective method for genetic transformation in the case of A. niger. A. niger cells are grown to the appropriate stage, harvested, and washed to remove any extraneous material before electroporation.(10) After that, the cells are resuspended in a solution containing the foreign DNA of interest and given a brief electrical pulse. After the cells have been allowed to recuperate, they are normally plated onto selective media to identify cells that have successfully incorporated the foreign DNA. Overall, electroporation is an effective tool for genetic manipulation in A. niger that can be used to investigate gene function, metabolic pathways, and other biological processes.(7)
Editing genes
Homologous recombination
Homologous recombination has been used successfully to modify the genome of A. niger, allowing for the production of novel enzymes with improved properties or the knockout of undesirable genes.(11) This method involves inserting a DNA molecule containing the desired modification into the A. niger cell, which then repairs the double-stranded break using its homologous DNA sequence. The modified cells can be easily identified and isolated by including a selectable marker in the introduced DNA.(8) The use of homologous recombination in A. niger gene editing has the potential to advance industrial biotechnology by allowing the development of more efficient and sustainable processes.
CRISPR-Cas system
CRISPR-Cas is a powerful gene editing tool that allows for precise DNA sequence modification. Many organisms, including the fungus Aspergillus niger, have used it successfully. The “CRISPR-Cas” system works by targeting a specific DNA sequence with a guide RNA molecule, which is then cut by the Cas enzyme. Natural DNA repair mechanisms in the cell can then be used to insert or delete specific genetic information at the site of the cut.(12) This system has been used in A. niger to improve enzyme and metabolite production by modifying specific genes involved in these processes. “CRISPR-Cas” has also been used to study gene function in A. niger, allowing researchers to gain a better understanding of the genetics of this important fungus. Overall, the “CRISPR-Cas” system in A. niger is a valuable tool for basic research as well as biotechnological applications.(7) Self-cleaving ribozymes are usually fused to the 5'- and 3'-ends of the gRNA to facilitate its effective release following transcription table 2.
|
Table 2. CRISPR-Cas application in A. niger hosts |
|||
|
Hosts |
Donor DNA |
Cas Expression |
Grna Expression |
|
A. niger SH2/ A. niger FGSC1279 |
- |
Codon-optimized SpCas9 with a D10A mutation; nucleus localization signal PKKKRKV fused at the C-terminus of Cas9; for converting cytidine to thymine, the rat cytidine deaminase, and uracil glycosylase inhibitor were fused to N-terminus and C-terminus of Cas9, respectively; Ptef1 and Ttef1; episomal expression. |
Pu6 and Tu6; episomal expression |
|
A. niger NIG81 |
Liner DNA; 60 bp homology arm. |
Codon-optimized Lachnospiraceae bacterium Cas12a; nucleus localization signal PKKKRKV fused atthe C-terminus of Cas12a;Ptef1and Ttef1; episomal expression. |
Pu3 and Tu3; episomal expression. |
|
A. niger N593/A. niger NRRL2270 |
Liner DNA; 600 bp homology arm. |
Codon-optimized SpCas9; nucleus localization signal SRADPKKKRKV fused at the C-terminus of Cas9; A. niger pyruvate kinase promoter and TglaA; episomal expression. |
The promoters and terminators of A. niger tRNAAla5,23, tRNAArg8,21, tRNAAsp2,5, TrnaCys1,2 tRNAAla5,23 tRNAGly13 tRNAHis2,3 tRNAI1e4,8 tRNALeu6,14 tRNALys6,17 tRNAMet4,9, tRNAPhe2,12 tRNAProl,3 tRNASer4,7, tRNAThr5,6 tRNATyr3,7, tRNAVal1,4,15. for benefiting the presice release of gRNA, tRNA fused at 5’-end of gRNA; episomal expression. Pu3andTu3 episomal expression. |
|
A. niger ATCC 1015 |
Liner or circular DNA; 35-1500 bp homology arm. |
Codon-optimized Streptococcus pyogenes Cas9 (SpCas9); nucleus localization signal PKKKRKV/ SRADPKKKRKV fused at C-terminus of Cas9; Aspergillus. nidulans tef1 promoter (Ptef1) and terminator (Ttef1)/ A. niger glucoamylase promoter (PglaA) and terminator (TglaA)/ A. niger coxA promoter and A. nidulans trpC terminator (TtrpC); episomal expression/integration expression/in vitro assembling ribonucleoprotein complex of Cas9 and gRNA. |
A. nidulansgpdA promoter (PgpdA) and TtrpC/ A. niger mbfA promoter and TtrpC/ Aspergillus fumigatus u3 promoter (Pu3) and terminator (Tu3); for benefiting the precise release of gRNA, hammerhead (HH) and hepatitis delta virus (HDV) ribozyme were fused to 5’- and 3’-ends of the gRNA/each gRNA was connected by tRNAgly; episomal expression/ integration expression/ in vitro assembling ribonucleoprotein complex ofCas9 and gRNA. |
|
A. niger G1 |
Liner DNA; 40 bp homology arm |
Codon-optimized SpCas9; nucleus localization signal PKKKRKV and KRPAATKKAGQAKKKK were fused at N- and C-terminus of Cas9,respectively; PglaA and TglaA; integration expression. |
Promoters of Homo sapiens u6, yeast u6, A. niger u6 or A. niger 5S rRNA, and an artificial terminator TTTTTT; integration |
|
A. niger CBS513.88 |
Liner DNA; 100 /500 bp homology arm |
Codon-optimized SpCas9; nucleus localization signal PKKKRKV fused at C-terminus or both ends of Cas9; Ptef1 and Ttef1/ PgpdA and TtrpCepisomal expression. |
PgpdA and TtrpC/ Aspergillus oryzae u6 promoter (Pu6) and terminator (Tu6)/ A. fumigatus u6 promoter and Tu6; for benefiting the precise release of gRNA, HH and HDV ribozyme were fused to 5’-end and 3’-end of gRNA, respectively; episomal or integration expression |

Figure 2. Strategies for improving recombinant protein expression in A. niger
Enzyme expression optimization in A. niger
To achieve maximum enzyme expression in A. niger, several parameters need to be taken into consideration.These include selecting appropriate promoter and terminator sequences, using appropriate growth conditions, and selecting appropriate expression vectors. Furthermore, optimizing codon usage, and gene copy number, and incorporating post-transcriptional regulatory elements like ribosome binding sites can improve enzyme expression levels. Finally, genetic engineering techniques like gene editing and gene silencing can be used to boost A. niger enzyme expression even further. Overall, a combination of these approaches may result in effective enzyme expression optimization in this fungus.
Cassette for expressing genes
Promoter
A gene expression cassette promoter is a DNA sequence that initiates transcription and controls gene expression. The selection of a suitable promoter is critical for efficient gene expression in Aspergillus niger, a filamentous fungus widely used in industrial fermentation processes. Strong constitutive promoters, such as glyceraldehyde-3-phosphate dehydrogenase (gpdA) and Aspergillus nidulanstrpC promoters, are commonly used in A. niger. Other inducible promoters have been used to regulate gene expression in response to specific nutrients, including the galactose-inducible gaaA promoter and the xylose-inducible xlnA promoter. Furthermore, the use of synthetic promoters to fine-tune gene expression levels has been investigated.(13) Overall, the choice of a suitable promoter in A. niger is determined by the desired level and timing of gene expression.
5’-untranslated region(5'-UTR)
(5'-UTR) of an Aspergillus niger gene expression cassette is a segment of DNA located upstream of the gene's coding region. This region influences the stability and translation efficiency of mRNA molecules, which helps to regulate gene expression. The 5'-UTR of A. niger has been found to contain cis-acting regulatory elements like promoter sequences, ribosomal binding sites, and mRNA stability elements. These elements are required for controlling the timing and level of gene expression, as well as ensuring that the gene product is produced in the appropriate amounts and at the appropriate time. Furthermore, the 5'-UTR can be engineered to boost the expression of heterologous genes, which is important for industrial biotechnology applications such as enzyme production or biofuel production.(14) In conclusion, the 5'-UTR of an A. niger gene expression cassette is an important component for controlling gene expression and optimizing protein production.(9)
Reading open frame
A DNA sequence known as an A. niger gene expression cassette permits the controlled expression of a desired gene in the fungus Aspergillus niger. A promoter region, which starts the gene's transcription, and a terminator sequence, which marks the end of transcription, are typically present in the cassette. Additionally, the cassette might include additional regulatory components like enhancers or silencers that can adjust the level of gene expression.The specific DNA section that encodes the desired protein is called the open reading frame (ORF), and it is located within the cassette.(14) Codon usage is typically adjusted, and the nucleotide sequence is made more effective for translation so that this ORF can be expressed in A. niger. The desired protein can then be effectively expressed by introducing the cassette into A. niger using methods like transformation or electroporation. These cassettes can be used for a variety of tasks, such as the creation of industrial enzymes or therapeutic proteins.(15)
Gene locus and copy number
A promoter, a gene of interest, and a terminator are all found in a DNA sequence known as a gene expression cassette, and they work together to control gene expression. Using homologous recombination, gene expression cassettes in A. niger can be incorporated(7) into the genome at particular loci. The choice of promoter and the method of integration can affect copy gene number, which refers to the number of copies of a specific gene that are present in a cell. For instance, integrating the cassette at a high-copy-number locus and using a powerful promoter can boost the level of gene expression.(15) Optimizing gene expression in A. niger for various biotechnological applications requires an understanding of these factors.
Procedures the following translation
Degradation of protein in the endoplasmic reticulum is reduced
A. niger's endoplasmic reticulum (ER) reduces protein degradation through several post-translational processes. One such procedure is the quality control mechanism, which entails the detection and elimination of improperly folded or incompletely put-together proteins. Chaperones and the ubiquitin-proteasome system play a role in this. Retrotranslocation of ER-associated degradation substrates to the cytosol, where they are destined for proteasomal degradation, is another process.(16) The recognition and destruction of terminally misfolded proteins in the ER are additional tasks of the ER-associated degradation pathway. Overall, these procedures support the preservation of the correct folding and operation of proteins in the ER and guarantee the caliber of the proteins secreted by A. niger.(11)
Improvement in protein export
Post-translational mechanisms can be used to enhance protein export in A. niger. One strategy is to modify the chaperone system or engineer the signal peptide sequence to improve the secretion pathway.(7) Another method is to overexpress chaperones or folding catalysts to improve the endoplasmic reticulum's (ER) ability to fold proteins. Protein secretion can also be increased by increasing the effectiveness of vesicle trafficking and fusion. The yield and quality of proteins secreted by A. niger can be increased by combining these techniques, making it a more productive and economical platform for protein synthesis.(17)
Impede the medium's degradation of proteins
Protein modifications known as post-translational processes take place after proteins have been created. A critical step in maximizing protein production in the case of A. niger is minimizing protein degradation in the medium.(18) The medium's pH and temperature can be optimized, protease inhibitors can be used, and the expression of chaperone proteins can be altered, among other things, to achieve this. Protein degradation can be kept to a minimum, increasing target protein yield and raising production process efficiency as a whole. The function and stability of the protein may also be impacted by post-translational processes that involve modifications like glycosylation or phosphorylation.(11,18)
Morphological design
Genetic engineering techniques for Morphology
Through genetic techniques like gene overexpression, regulation, and knockouts, A. niger's morphology can be altered. Genes that are responsible for undesirable morphology can be removed using gene knockout techniques, whereas overexpressed genes can improve desired traits.(16) Controlling morphological features can also be accomplished by regulating gene expression. Additionally, specific genes can be targeted and altered for desired morphological changes using synthetic biology tools like CRISPR/Cas9. These genetic techniques can be used to modify A. niger's morphology for increased production of targeted compounds or other industrial uses.(19)
A correlation between morphology and the production of enzymes
Morphological engineering is the practice of modifying the morphology of microorganisms to enhance their functionality in a variety of applications, such as the production of enzymes. The morphology of the mycelium has been discovered to be closely correlated with enzyme production in the case of Aspergillus niger, a filamentous fungus that is frequently used for industrial enzyme production.(20) The formation of short, compact, highly branched mycelia with short hyphal compartments has been shown to increase the production of extracellular enzymes, whereas long, sparsely branched mycelia with large compartments have been shown to decrease enzyme production. These findings have significant ramifications for fermentation process design and optimization for A. niger enzyme production via morphological engineering.(15)
Techniques for morphological engineering using bioprocesses
By changing the morphology of the microorganism, morphological engineering is a bioprocess technique used to increase the production of metabolites. This is possible with A. niger through genetic engineering as well as environmental modification techniques like adjusting pH, temperature, and nutrient availability.(4) For instance, creating stress conditions can cause hyphae to form, increasing surface area and nutrient uptake. Additionally, the deletion of genes involved in the synthesis of cell walls can result in the development of larger and more intricate hyphae. It has been demonstrated that these techniques help A. niger produce more crucial metabolites like citric acid and glucoamylase.(21)
Niger mycotoxin production
Aspergillus niger is a common fungus found in soil, air, and rotting plant material. It has been found to produce a wide range of secondary metabolites, including mycotoxins. Mycotoxins are hazardous chemicals that can injure humans and animals if consumed. Mycotoxins produced by A. niger include ochratoxin A, citrinin, and fumonisin.(22) Ochratoxin A is a highly toxic toxin that can induce kidney damage and may be carcinogenic. Citrinin has been demonstrated in animal studies to be nephrotoxic and hepatotoxic, whereas fumonisins are known to induce neural tube abnormalities and other health issues in livestock.(11) Environmental factors such as temperature, pH, and nutrient availability influence A. niger's generation of mycotoxins.
Formation of mycotoxin
Aspergillus niger is a type of fungus that can be found in soil, plants, and food. It is known to produce mycotoxins such as ochratoxin A, fumonisins, and citrinin, which can contaminate food and feed and endanger human and animal health. Temperature, humidity, substrate composition, and environmental variables all have an impact on A. niger mycotoxin synthesis. Mycotoxins are produced by A. niger by secondary metabolite biosynthesis, which can occur in fungal hyphae or spores. Some research suggests that A. niger can develop mycotoxins even when no visible mold growth is present, emphasizing the significance of monitoring for mycotoxin contamination in food and feed.(22,23) A. niger mycotoxins and other significant secondary metabolites were shown in figure 3.
Elimination of Mycotoxin
Aspergillus niger is a fungus that can create mycotoxins like ochratoxin A, which can taint food and cause health issues. It is critical to control the circumstances under which A. niger thrives, including temperature, humidity, and nutrient availability, to eliminate mycotoxin production. This can be accomplished by safe food storage and handling, as well as regular contamination monitoring. Furthermore, numerous physical, chemical, and biological procedures, such as washing, heating, and treatment with enzymes or microbes, can be employed to remove mycotoxins from contaminated objects. Effective mycotoxin eradication necessitates a multifaceted approach that includes techniques for prevention, detection, and remediation.
Mycotoxin-related gene deletion
Aflatoxins are mycotoxins generated by the fungus Aspergillus niger that can contaminate food and feed, putting human and animal health at risk. Aflatoxins can be reduced by deleting the genes responsible for their generation in A. niger. This is possible using genetic engineering tools such as CRISPR/Cas9.(24) A strain of A. niger that lacks the aflatoxin biosynthesis genes is unable to manufacture these poisons and thus cannot contaminate food and feed. This method has the potential to lower the danger of aflatoxin contamination while also improving food safety.(21)
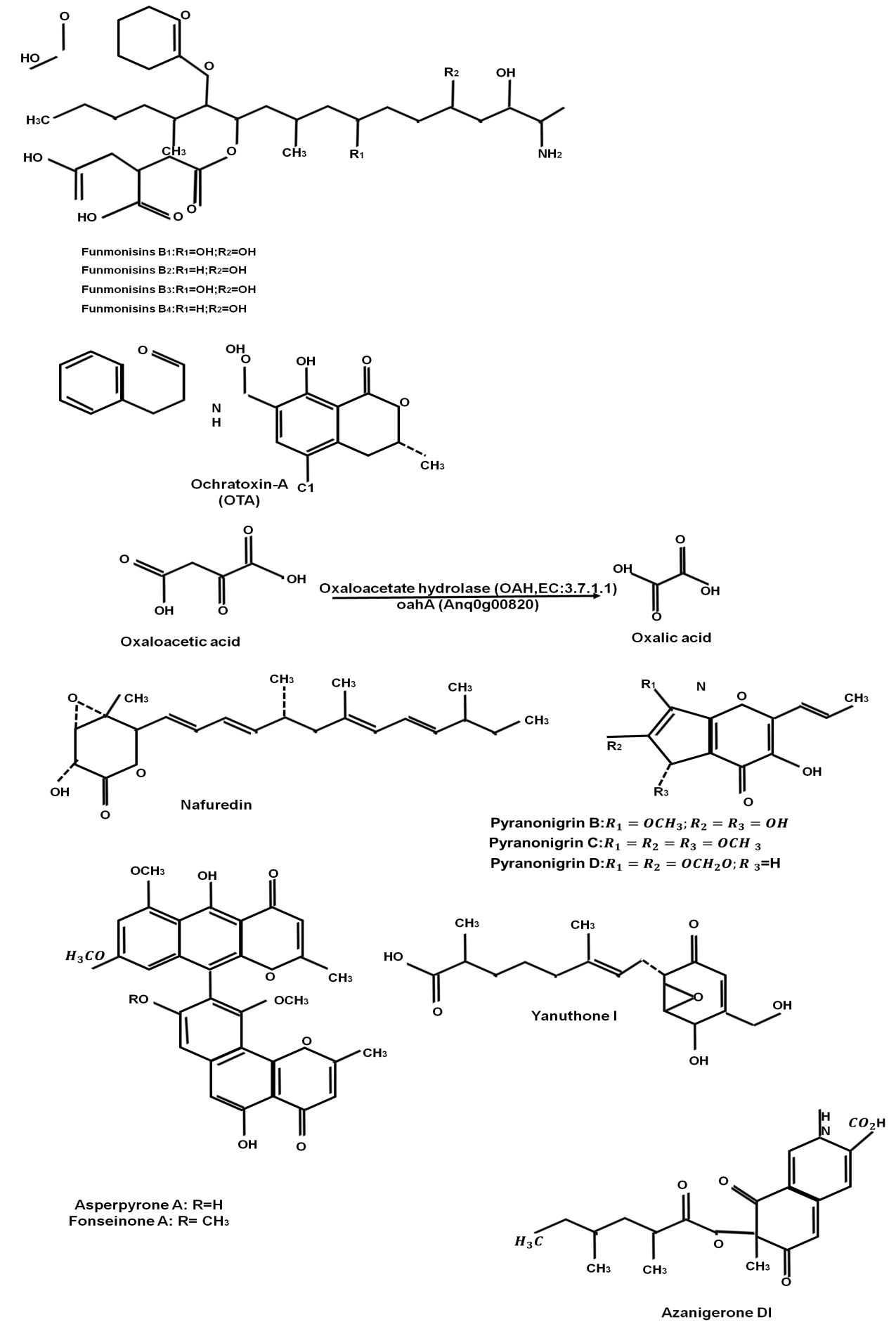
Figure 3. A. niger mycotoxins and other significant secondary metabolites
Enhancing fermentation conditions
The filamentous fungus known as A. niger is frequently exploited for the production of a wide variety of metabolites, including mycotoxins. Optimizing fermentation conditions is critical for increasing mycotoxin generation. pH, temperature, agitation, aeration, carbon and nitrogen sources, and trace elements all play important roles in regulating A. niger growth and metabolite production. Mycotoxin generation has been observed to be enhanced by pH values of 4-6, a temperature of 25-30°C, and adequate agitation and aeration levels.(25) Mycotoxin yield can also be increased by optimizing carbon and nitrogen sources such as glucose and ammonium sulfate. Furthermore, trace metals like copper and iron can greatly boost mycotoxin formation in A. niger.
CONCLUSION
A. niger is a common filamentous fungus utilized for food enzyme synthesis due to its higher protein secretion efficiency, low medium costs, and safety. The “CRISPR-Cas” technology has considerably enhanced the efficiency of gene editing in A. niger. To build a very cell factory of robust for making food enzymes, optimization technologies like gene expression cassettes, and post-translation procedures have been devised. The production of mycotoxin by A. niger can be decreased by deleting genes associated with the production of mycotoxin and improving the environment under which fermentation occurs. Researchers used the “CRISPR-Cas” system to find the orthologs of Cas9 and Cas12 and then produced mutants of Cas9 and Cas12 with extra PAM. This allowed them to expand the region of the A. niger genome that could be edited. To promote the production of heterogeneous proteins in A. niger, introns can be introduced, and codon use must be optimized. However, genetic engineering directed targeting A. niger's protein secretory system is now limited to protein folding, degradation, and membrane vesicle trafficking. To boost export efficiency, more protein export pathways should be identified and regulated in the future, screening robust A. niger hosts or expression components will need for methods of directed evolution that are founded on ultra-high-throughput technologies.
REFERENCES
1. García MV, Parussolo G, Moro CB, Bernardi AO, Copetti MV. Fungi in spices and mycotoxigenic potential of some Aspergilli isolated. Food Microbiol. 2018;73:93-98.
2. Kluge J, Terfehr D, Kück U. Inducible promoters and functional genomic approaches for the genetic engineering of filamentous fungi. Appl Microbiol Biotechnol. 2018;102:6357-6372.
3. Huang L, Dong L, Wang B, Pan L. The transcription factor PrtT and its target protease profiles in Aspergillus niger are negatively regulated by carbon sources. Biotechnol Lett. 2020;42:613-624.
4. Zheng X, Zheng P, Zhang K, Cairns TC, Meyer V, Sun J, Ma Y. 5S rRNA promoter for guide RNA expression enabled highly efficient CRISPR/Cas9 genome editing in Aspergillus niger. ACS Synth Biol. 2018;8(7):1568-1574.
5. Park HS, Jun SC, Han KH, Hong SB, Yu JH. Diversity, application, and synthetic biology of industrially important Aspergillus fungi. Adv Appl Microbiol. 2017;100:161-202.
6. Aguilar-Pontes MV, Brandl J, McDonnell E, Strasser K, Nguyen TTM, Riley R, Mondo S, Salamov A, Nybo JL, Vesth TC, Grigoriev IV. The gold-standard genome of Aspergillus niger NRRL 3 enables a detailed view of the diversity of sugar catabolism in fungi. Stud Mycol. 2018;91(1):61-78.
7. Yin X, Shin HD, Li J, Du G, Liu L, Chen J. P gas, a low-pH-induced promoter, as a tool for dynamic control of gene expression for metabolic engineering of Aspergillus niger. Appl Environ Microbiol. 2017;83(6):e03222-16.
8. Zheng X, Cairns T, Zheng P, Meyer V, Sun J. Protocol for gene characterization in Aspergillus niger using 5S rRNA-"CRISPR-Cas"9-mediated Tet-on inducible promoter exchange. STAR Protoc. 2022;3(4):101838.
9. Volokhina I, Gusev Y, Mazilov S, Moiseeva Y, Chumakov M. Computer evaluation of VirE2 protein complexes for ssDNA transfer ability. Comput Biol Chem. 2017;68:64-70.
10. vanLeeuwe TM, Arentshorst M, Ernst T, Alazi E, Punt PJ, Ram AF. Efficient marker-free CRISPR/Cas9 genome editing for functional analysis of gene families in filamentous fungi. Fungal Biol Biotechnol. 2019;6(1):1-13.
11. Ding Y, Wang KF, Wang WJ, Ma YR, Shi TQ, Huang H, Ji XJ. Increasing the homologous recombination efficiency of eukaryotic microorganisms for enhanced genome engineering. Appl Microbiol Biotechnol. 2019;103:4313-4324.
12. Vanegas KG, Jarczynska ZD, Strucko T, Mortensen UH. Cpf1 enables fast and efficient genome editing in Aspergilli. Fungal Biol Biotechnol. 2019;6:1-10.
13. Kiesenhofer DP, Mach RL, Mach-Aigner AR. Influence of cis element arrangement on promoter strength in Trichoderma reesei. Appl Environ Microbiol. 2018;84(1):e01742-17.
14. Andreeva I, Belardinelli R, Rodnina MV. Translation initiation in bacterial polysomes through ribosome loading on a standby site on a highly translated mRNA. Proc Natl Acad Sci U S A. 2018;115(17):4411-4416.
15. Leynaud-Kieffer LM, Curran SC, Kim I, Magnuson JK, Gladden JM, Baker SE, Simmons BA. A new approach to Cas9-based genome editing in Aspergillus niger that is precise, efficient, and selectable. PLoS One. 2019;14(1):e0210243.
16. Huang L, Dong L, Wang B, Pan L. The transcription factor PrtT and its target protease profiles in Aspergillus niger are negatively regulated by carbon sources. Biotechnol Lett. 2020;42:613-624.
17. Sun X, Su X. Harnessing the knowledge of protein secretion for enhanced protein production in filamentous fungi. World J Microbiol Biotechnol. 2019;35:1-10.
18. Schmideder S, Barthel L, Müller H, Meyer V, Briesen H. From three-dimensional morphology to effective diffusivity in filamentous fungal pellets. Biotechnol Bioeng. 2019;116(12):3360-3371.
19. Kamaruddin N, Storms R, Mahadi NM, Illias RM, Bakar FDA, Murad AMA. Reduction of extracellular proteases increased activity and stability of heterologous protein in Aspergillus niger. Arab J Sci Eng. 2018;43:3327-3338.
20. Fiedler MR, Cairns TC, Koch O, Kubisch C, Meyer V. Conditional expression of the small GTPase ArfA impacts secretion, morphology, growth, and actin ring position in Aspergillus niger. Front Microbiol. 2018;9:878.
21. Willemse J, Büke F, van Dissel D, Grevink S, Claessen D, van Wezel GP. SParticle, an algorithm for the analysis of filamentous microorganisms in submerged cultures. Antonie Van Leeuwenhoek. 2018;111:171-182.
22. Frisvad JC, Møller LL, Larsen TO, Kumar R, Arnau J. Safety of the fungal workhorses of industrial biotechnology: update on the mycotoxin and secondary metabolite potential of Aspergillus niger, A. oryzae, and Trichoderma reesei. Appl Microbiol Biotechnol. 2018;102:9481-9515.
23. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos MDL, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J. Safety and efficacy of alpha-amylase from Bacillus amyloliquefaciens DSM 9553, Bacillus amyloliquefaciens NCIMB 30251, A. oryzae CBS 585.94, and A. oryzae ATTC SD-5374, endo-1, 4-beta-glucanase from Trichoderma reesei ATCC PTA-10001, Trichoderma reesei ATCC SD-6331 and Aspergillus niger CBS 120604, endo-1, 4-beta-xylanase from Trichoderma koningii MUCL 39203 and Trichoderma citrinoviride CBS 614.94 and endo-1, 3 (4)-beta-glucanase from Aspergillus tubingensis MUCL 39199 as silage. EFSA J. 2018;16(4):e05224.
24. Tilburn J, Scazzocchio C, Taylor GG, et al. Transformation by integration in Aspergillus nidulans. Gene. 1983;26(2-3):205-221.
25. Walaszczyk E, Podgórski W, Janczar-Smuga M, Dymarska E. Effect of medium pH on chemical selectivity of oxalic acid biosynthesis by Aspergillus niger W78C in submerged batch cultures with sucrose as a carbon source. Chem Pap. 2018;72:1089-1093.
FINANCING
No financing.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: Renuka Jyothi Shettru, Divya Shrivastava, Sudhir Singh.
Research: Renuka Jyothi Shettru, Divya Shrivastava, Sudhir Singh.
Writing - proofreading and editing: Renuka Jyothi Shettru, Divya Shrivastava, Sudhir Singh.