REVIEW
A Comprehensive Review of the Pathogenesis and Virulence Factors of E. coli
Revisión exhaustiva de la patogénesis y los factores de virulencia de E. coli
Asha Kademane1 ![]() *,
Meenal Dixit2
*,
Meenal Dixit2 ![]() , Vasundhara3
, Vasundhara3 ![]()
1Department of Life Science, School of Sciences, JAIN (Deemed-to-be University). Bangalore, India.
2School of Life and Basic Sciences, Jaipur National University. Jaipur, India.
3Department of Microbiology, Teerthanker Mahaveer University. Moradabad, Uttar Pradesh, India.
Cite as: Kademane A, Dixit M, Vasundhara. A Comprehensive Review of the Pathogenesis and Virulence Factors of E. coli. Salud, Ciencia y Tecnología. 2023;3:411. https://doi.org/10.56294/saludcyt2023411
Received: 20-04-2023 Reviewed: 13-05-2023 Accepted: 16-06-2023 Published: 17-06-2023
ABSTRACT
Escherichia coli are very adaptable microbes that play a significant role in the typical gut microbiota of both humans and animals. This non-pathogenic commensal bacterium has the ability to acquire a variety of mobile genetic components that are comprehensive and include genes for virulence factors is a newly discovered human pathogen that may cause a wide range of extraintestinal and gastrointestinal illnesses. Nine distinct enteric E. coli pathotypes, which range from different gastrointestinal illnesses to infections of the urinary tract, have been thoroughly characterised. These collaboration use a variety of virulence factors and effectors that regulate their transmission and pathogenicity by disrupting the functioning of host cells. This article highlights recent advances in our knowledge of the many viruses linked to the genes that distinct external ecological of gastric harmful E. coli employ to trigger extraintestinal and digestive issues in people.
Keywords: Pathogenic Enteric E. Coli; E. Coli Pathotypes; Genes for Virulence Factors.
RESUMEN
Escherichia coli es un microbio muy adaptable que desempeña un papel importante en la microbiota intestinal típica de humanos y animales. Esta bacteria comensal no patógena tiene la capacidad de adquirir una variedad de componentes genéticos móviles que son completos e incluyen genes para factores de virulencia es un patógeno humano recientemente descubierto que puede causar una amplia gama de enfermedades extraintestinales y gastrointestinales. Se han caracterizado a fondo nueve patotipos distintos de E. coli entérica, que abarcan desde distintas enfermedades gastrointestinales hasta infecciones de las vías urinarias. Estos colaboran con diversos factores de virulencia y efectores que regulan su transmisión y patogenicidad alterando el funcionamiento de las células del huésped. En este artículo se ponen de relieve los recientes avances en nuestro conocimiento de los numerosos virus vinculados a los genes que emplean las distintas ecologías externas de E. coli patógenas gástricas para desencadenar problemas extraintestinales y digestivos en las personas.
Palabras Clave: E. Coli Entérica Patógena; Patotipos De E. Coli; Genes de Factores de Virulencia.
INTRODUCTION
Escherichia coli, a cylindrical, gram- positive member of the organism’s family of bacteria, was first discovered and identified in new-born feces. E. coli begin to colonize and live in new-borns’ gastric tracts a several moments after arrival. Common E. coli genotypes and people cohabit having any unfavorable effects, which has multiple benefits that are mutual. Yet, in individuals with impaired immune systems or infected hosts, commensal E. coli may lead to illness. Particularly, specific E. coli have a role in a number of illnesses, involving extra intestinal and digestive ailments in both people and animals across the globe. Seven pathovars of E. coli strains isolated from individuals that produce diarrheagenic and extra intestinal disorders were identified. Among those, intestinal pathogenic E. coli has been associated with seven different route categories, involving enter pathogenic “E. coli (EPEC)”, “Enter haemorrhagic E. coli (EHEC)”, “Enter toxigenic E. coli (ETEC)”, “Enter invasive E. coli (EIEC)”, “Enter aggregative E. coli (EAEC)”, “Diffusely adherent E. coli (DAEC)”, and, a new path type, “Adherent-Invasive E. coli (AIEC)”, primarily producing diarrhea with digestive issues. Yet, the extra intestinal disorders Hemolytic Uremic Syndrome (HUS) and others have been linked to the EHEC path type. As pathogens transmitted via food these resulted to many deadly breakouts among developed as well as underdeveloped countries, numerous of these specific field are causing medical problems. There are several illnesses that enteric E. coli path types are linked to via completely diverse etiology. The process by which microorganisms create illness or dysfunction, often through exhibiting toxicity, is known as pathophysiology. Genetics that encode –factors.(1,2)
This condition, fish tank granuloma, Searls ulcer, and tuberculosis (TB) are just a few of the illnesses that Mycobacterium species (spp) may cause. One of the most significant public health issues, TB is brought on by the bacteria Mycobacterium tuberculosis (Mtb), and it may result in high rates of morbidity and death around the globe.(2,3,4) According to the WHO's 2018 TB report, TB continued to be a significant worldwide health problem in 2017, with an estimated 1,3 million HIV-negative persons dying from the disease. Additionally, there were an additional 300 000 TB-related deaths among HIV-positive individuals. Additionally, it was anticipated that there were 10,0 million new cases of TB globally, or 133 instances for every 100 000 people. Identifying a different strategy for the prevention and cure of TB is thus absolutely crucial. The swift spreading of diseases illnesses and the worrying rise of drug resistance, particularly The need for effective new drugs has been raised by the advent of multidrug-resistant (MDR-TB) and extensively drug-resistant (XDR-TB) types of mycobacteria.(3)
Pathogenic Escherichia coli (E. coli) are a class of healthy anaerobic that, when combined with other virulence factors such as adhesins, invasins, toxins, and capsules, may harm healthy people. According to medical, epidemiological, and infectiousness behaviors, there are six different pathotypes of pathogenic E. coli: “enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAEC), diffusely adherent E. coli (DAEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), and enterohemorrhagic E. coli (EHEC)”. Korean occurrences of infectious E. coli are mostly caused by EPEC (60,5 %), followed by ETEC (31,2 %), EHEC (6,8 %), and EIEC (1,5 %).(6) One of these, EHEC, may induce diarrhea by the attaching-effacing (A/E) lesions mechanism with only a little infectious dose (1-100 CFU). Strong acid tolerance in EHEC enables it to survive in foods with low pH. 16 cases of E. coli O157:H7 Phage Type 49 were linked to the eating of a locally manufactured yogurt in the southwest the 10-day survival of E. coli O157:H7 was shown in both conventionally infected yogurt and was before the-hydrolyzed yogurt, while it was found to live for 22 days in lactose-free yogurt. After 28 days, E. coli strains that are present O157:H7 in Greek yogurt barely dropped by around 1,4 log CFU/g.(4)
Individual’s gastric tissue cells line the small intestine track, acting as an obstruction preventing bacteria. The glycocalyx is an intracellular web of particles rich in carbohydrates that are connected to cell membranes or are expelled by organisms into the surroundings, coats the mucosal barrier. Mucosal surfaces vary in thickness from 300 micrometers in the stomach to 700 micrometers in the intestines. The gastrointestinal fluids contains a number of protective substances that act as barriers to movement. The symbiotic bacterium utilizes nutritional value of mucus cellulose, which are made accessible using proteins that degrade glycans, and it is found in the mucosa barrier's outermost layers.(5,12)
Antibiotic resistance (AMR) is a natural occurrence associated with the emergence and dissemination of resistance genes amplified by antibiotic use. The One Health strategy highlights the significance of AMR as a significant issue for both public health and food animal production systems. E. coli is one of the pathogens that is increasingly important, and it has been hypothesized that both commensal and externally sourced infectious Antimicrobial genes that might be transferred to pathogenic or opportunistic bacteria are sometimes stored in E. coli populations. Particularly, worrying novel susceptibility to antibiotics elements are apparently being acquired by ExPEC strains. ExPEC infections are currently a severe public health hazard globally because to the fast rising global multidrug susceptibility and a dwindling antibacterial arsenal to combat resistant bacteria. However, the clinical and financial effects of these illnesses and their best treatment are difficult, and as a result, both healthcare professionals and the general public are becoming more aware of the significance of ExPECs.(6)
In Aditya et al.(7), the highly antigenic T3SS structural protein EscC, which is shared by EHEC and other enteropathogenic microbes, was transported using phage P22 nanocontainers. EscC protein was successfully incorporated into P22 nanocontainers in a stable manner. Mice were intranasally vaccinated with the EscC-P22 particles, which led to the production of particular antibodies against EscC. Those antibodies demonstrated operational efficacy by increasing the phagocytic activity of murine macrophages infected with EHEC in vitro and decreasing bacterial adhesion to Caco-2 epithelium cells in vitro. A possible nanovaccine option for immunizing against EHEC O157:H7 infections is made up of EscC-P22-based atoms.
In Desvaux et al.(8), they sought to use metabolites, synbiotics, and live probiotics to limit the development of EHEC in an artificial ruminant environment utilizing collected rumen fluids (RFs). RF that had been inoculated with EHEC was given live Lactobacillus casei wild-type (LCwt), LCwt+PF (LCwt+PF with 0,5 % groundnut powder), an engineered LC that could overexpress linoleate isomerase (LCCLA), and their metabolites were obtained in molecule-free colony supernatants (CFCSwt, CFCSwt+PF, and CFCSCLA) at various times. All of the CFCS had a growth-stimulating impact on Lactobacillus spp., however the EHEC was repressed. Only LCwt+PF, as compared to other therapies, 72-hour reduction of 2,68 logs in EHEC. Metataxonomic study added credence to this finding. At 48 h, the existence of CFCSs was shown to reduce Bacteroidetes and Proteobacteria while increasing Firmicutes species in comparison to the control. Our findings imply that probiotic-derived compounds alter ruminant microbiome in a way that might be utilized to stop spreading of illnesses that affect cattle, notably EHEC.
In Yekani et al.(9), a significant infectiousness characteristics of E. coli are discussed, such as acidic obstructions, several types of bonding proteins like filaments, Outside cell proteins A, fibrillae, curli, kind III secretory infrastructure, the enzyme alkaline phosphatase produced by the PhoA genes in E. coli, and the disruption of eukaryotes signals by pathogens via injecting virulence proteins into the host cell cytoplasm. E.coli Therefore, the current review piece calls for innovative study on several E. coli virulence variables in order to implement integrated prevention methods.
In Saeedi et al.(10), enterotoxigenic Escherichia coli (ETEC) is discussed as a significant contributor to pediatric dysentery and visitors from underdeveloped nations. ETEC is distinguished by its capacity to create important virulence factors, such as enterotoxins and colonization factors (CFs), which connect to certain targets inside epidermal cells and cause dysentery. The microbial flora of the digestive system is a reliable and complex ecology that serves the host in a variety of ways, includes guarding it towards disease invasion.
Tosif et al.(11) identify the virulence indicators and contributors in multidrug resistant (MDR) isolates of E. coli on individuals in Lafia, Nigeria who had digestive and urinary tract diseases. Patients' urine and stool samples were collected (150 of each), and germs have been separated utilizing a spreading surface, with specimens method. After determining which infections of E. coli were sensitive to antimicrobial agents, virulence factors and genes imparting virulence were assessed. E. coli was present in 33,3 % of urine samples and 35,3 % of stool samples, 42 of the isolates were multidrug resistant. Every isolation had the ability to create propane and hemoglobin, while isolate U6 generated the greatest quantity of hemoglobin and the additional separates generally generated adequate amounts of pyrogen. All the infections also had cellular hydrophobia at >1,5 molecule sulfuric acid concentrations.
DEVELOPMENT
Adherent Invasive Escherichia coli (AIEC)
AIEC predominantly affects the human small intestine and is among the greatest significant causal systemic inflammation diseases, including IBD and CD. However, this path type could also exist in healthy persons' typical gut microbiota and not be a disease-causing factor. Idiotic IBD has no known primary etiology, according to studies done on the subject. With addition to AIEC, additional enteric infections such Escherichia species, Mycobacterium paratuberculosis, and Cytomegalovirus have been implicated with IBD and CD. The AIEC pathotype is an enteric pathogen that has the ability to attach to, penetrate, and grow inside of macrophages and epithelial cells.(13,14) The AIEC strains lack any specific virulence factors identified in other E. coli pathotypes. Three steps make up the development of AIEC: attachment, assault, and proliferation inside the body cells of the epithelium. The ileum's epithelial host cells must first be adhered to by CAECAM6, which is shown on these particular cells. Especially, the colonization of AIEC causes bowel irritation and a rise in CAECAM6 transcription in CD patients as a result of the activation of TNF- generation. After adhering to the epithelium hosts, the AIEC strain expresses and uses a number of virulence factors, including extended polarity fibers, exterior membrane vessels, and outside protein molecules, to facilitate assault, infection, and replication inside the phagocytes. The harmful contact with the intestinal mucosa is mediated by a number of viral factors and related generating regions. In AIEC alone for those who have CD, IBD, and ulcerous colitis, greater amounts of activity in genomes that code for adherence, an invading and survival-related viruses. The invasion protein ibeA, the invading proteins ipaH, the assault genome antibody, and virulence-related that regulate The kpsMT, yjaA, ibeA, ipaH, fyuA, and fimH II genes encode for ferric yersiniabactin absorption and encapsulation formation accordingly, are all found in AIEC collected from individuals who have digestive problems. Patients with IBD and CD may benefit from using AIEC strains as a novel treatment target. More chromosomal research is necessary, particularly to identify the genes that encode virulence factors, since multiple components of its pathophysiology remain unclear.
Diffusely Adherent Escherichia coli (DAEC)
DAEC is recognized as a heterogeneous E. coli pathotype that affects Hep-2 and HeLa cells in a diffuse fashion. In addition to difficulties during pregnancy, DAEC is linked to digestive system flora microbiota in both grownups and kids as well as youngsters between the ages of 1,5 and 5 who have dysentery. Younger children may get more chronic watery diarrhea brought on by DAEC. Adults who have DAEC strains linked to various chronic inflammatory intestinal disorders, such as Crohn's, celiac, and inflammation of the colon, are asymptomatic carriers. The phylogenetic group B2 has been include the DAEC strains, and it is important to note that this group dominates the human commensal E. coli strains. No adults had diarrhea considering the infection being isolated from feces and intestinal cultures. The pathogen's adhesion to certain host cells marks the beginning of the DAEC pathophysiology. The ability of DAEC strains to adhere to intestinal and urinary skin cells permits them to withstand elimination by digestion and micronutrition, accordingly. Additionally, the connection enables DAEC to communicate with the host cell, distribute and discharge pollutants, and initiate signalling processes in the host cell. Afa/Dr adhesins mediate DAEC's non-classical sequences of attachment and adhesion to the host cells.(15,16)
Although Afa/Dr adhesins play a major function in pathophysiology of DAEC varieties, same pathotype also secretes certain SPATE toxins. Two primary categories of SPATE poisons are released by Afa/Dr DAEC strains: class I, which has cytotoxic properties toward epithelium cells and includes An external cysteine that enzyme, a genes-encoded poison, a secretory autotransporter poison, and sigA poisons are all category II, non-harmful poisons that are produced by the sat, pet, EspP, and sigA genes, accordingly. DAEC isolates are also currently demonstrated to possess additional virulent factors, such types of pili, pilus adhesin, and heat-unstable enteroaggregative poisons, which are expressed through the pap, fim, and astA genes, etc.
Enteroaggregative Escherichia coli (EAEC)
In both industrialized and developing nations, EAEC has been identified as a new foodborne pathogen that is mostly responsible for acute and chronic infant dysentery as well as development retardation. In industrialized and EAEC also ranks session among emerging economies. The most common causes of severe and ongoing sickness for travelers after ETEC, and it is a significant contributor to Infections of the digestive tract in HIV/AIDS patients. Tenesmus, borborygmi, disorders and diarrhea are additional signs of EAEC infection. As was previously reported, in 2011 a combination EAEC/STEC outbreak (serotype O104: H4) led to a significant epidemic in Germany that over 4400 instances as a consequence of diarrhea and 50 fatalities. EAEC may effectively cause intestinal irritation and enteric colonization, which might enhance the potency of additional infectious microbes, according to a number of studies. EAEC often produces watery diarrhea, which may sometimes be mixed with blood or mucus. The colon may become mildly to severely inflame as a result of EAEC colonizing the small and large bowel mucosa. A "stacked-brick" pattern of adhesion to Hep-2 cells has historically been used to describe EAEC. Because EAEC is heterogeneous, the pathogenic processes of this disease are quite intricate. The three processes in the progression of EAEC include adhesion to the intestinal epithelium by adhesion aggregative filaments, biofilm creation, and the release of poisons that cause inflammatory conditions and apoptotic destruction.
In the present investigation, 11/66 (49 %) of the isolates were classified as aEPEC (positive eae gene alone), 19/66 (31 %) as tEPEC (positive eae gene+ bfpA gene), and 31/66 (18 %) as EAEC (positive pCVD432 gene). However, co-infection was present in 5/66 (9 %) of the isolates (tEPEC + EAEC in 4 isolates and aEPEC + EAEC in 1 isolate). The research isolates did not include any EHEC, ETEC, or EIEC (figure 1). Table 2 shows the distribution of DEC types by age groups. DEC prevalence found in stool samples. Diarrheal-causing E. coli are DEC, enteroaggregative E. coli (tEPEC), typical enteropathogenic E. coli (aEPEC), enteroinvasive E. coli (EIEC), enterohemorrhagic E. coli (EHEC), and enterotoxigenic E. coli (ETEC).
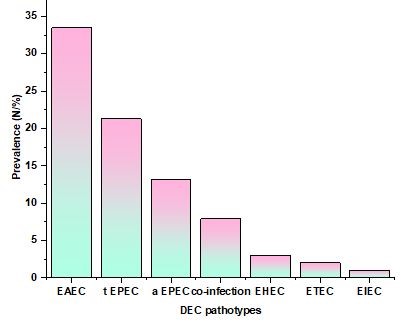
Figure 1. Prevalence (N %)
|
Table 1. DEC Pathotypes and Prevalence |
|
|
DEC Pathotypes |
Prevalence (N/%) |
|
EAEC |
33,49 |
|
t EPEC |
21,31 |
|
a EPEC |
13,18 |
|
co-infection |
7,9 |
|
EHEC |
3 |
|
ETEC |
2 |
|
EIEC |
1 |
Enteroinvasive Escherichia coli (EIEC)
When multiple kinds of Shigella and EIEC, which are invasive pathogenic to food, produce the infection or shigellosis, the usual clinical symptoms include intense ulceration and gastrointestinal bleeding, stomach pains, and temperature. Shigella, formerly known as Bacillus dysenteriae, was first identified by Kiyoshi Shiga in 1897 in the nations during a severe outbreak that resulted in over 91 000 cases and 20 % death. EIEC was identified and given the identical infectious, biological, and physiological characteristics as Shigella fifty years later. They may thus be grouped under a single pathovar. Considering the medical value of Shigella organisms, nonetheless, a clear differentiation continues to exist. Shigella and EIEC are both non-motile, lysine decarboxylase-negative, and incapable of fermenting galactose. These characteristics are thought to set Shigella species and EIEC apart from more microorganisms. Due to the fact that it is a persistent cellular pathogen that lacks either adhesion or flagella variables, EIEC differentiates from different pathovars of E. coli. Four different species of S. dysenteriae, S. sonnei, S. flexneri, S. boydii, and are among the variety of that may produce different types of diarrhea as well as chronic esophageal and additional-intestinal illnesses in people. Invasion inflammation colon and loose stool are the main symptoms of EIEC genotypes.through employing impactors of proteins to stay and live within the colonocytes, EIEC inhibits the host immunological response and undermines immunological protection once localised in the nucleus of epithelial cells. Genetic stimulation and expression of NF-êB are inhibited by OspF and OspG. IpaH9,8 and OspB also inhibit the production of pro- mediators that cause inflammation, such as interleukin-8, OspZ, OspI, and IpaH4,5 are additional effectors that assist EIEC in downregulating and dampening reactions of inflammation. Furthermore, by storing the VirG and disrupting micro tubules accordingly, receptors IcsB and VirA encourage organize-cell-autophagy-mediated destruction in EIEC. Shigella enterotoxins 1 and 2, which are encoded by the ShET1 and ShET2 genes, accordingly, are among other pathogenicity variables that are linked to frequent symptoms of watery diarrhea in EIEC and Shigella infections. ShET2 has been discovered on a sensitivity plasmid, while ShET1 is identified on the susceptibility area and a chromosomal region. The function of the secretory intestine is influenced by both enterotoxins. In the gastrointestinal tissue of the host, ShET2 causes inflammatory. In addition, EIEC and Shigella species produce and release additional toxins that other pathogenicity component genetics, like Pic, encoding. SepA, SigA, and Sat. Pic controls the expression of ShET1 and has been encoded by chromosomal location . Toxins produced by the Enterobacteriaceae (SPATEs) family of this enzyme autotransporters includes the genes for the SepA and Pic toxin. SPATEs are trypsin-based toxins that are often produced involving virulent strains of E. coli and Shigella and are delivered through autotransporter routes. In the EIEC or Shigella-infected animals, the SigA-encoded possible exposure aids in the buildup of intestinal fluid. Other virulence factors, such as those involved in the production of aerobactin, Pathogenicity component transcription, pathogenicity element protein synthesizing, suppression of the immune system cell transport organs, Complicated spores ferrous receptors, SPATE toxin, and the restriction of inflammatory cells, were just discovered and discovered in abundance in EIEC and Shigella isolated cells.
Stx1, Stx2, and invA did not provide any beneficial outcomes, whereas aggR was the most common VF in both clinical patients and controls. Overall, clinical cases had a greater percentage of positive isolates to any VF (isolates in which at least one VF was identified) than did control E. coli, while differences were only statistically significant (P = 0,034) in the case of est (table 1). Between the three hospitals in the case individuals, there were substantially different proportions of isolates that were aggR and est positive (P 0,001 and P = 0,010, respectively). EAEC was found in 36,7 and 33,3 % of the clinical cases and control participants, respectively, based on the VF profile. EAEC/ETEC (8,37 %) was the most frequent combination of hybrid pathotypes, which were found in 14,1 % of the population (clinical patients had a substantially higher rate of hybrid pathotypes, 15,8 %, compared to the control group's 3,0 %, P = 0,048) (table 2). In contrast to 23,26 % of the clinical cases, samples negative to all VFs (isolates not categorised into pathotypes) were identified in 45,5 % of the controls (figure 2).
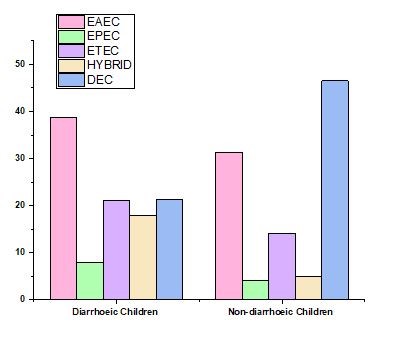
Figure 2. DEC path type dispersion in cases as well as control patients
|
Table 2. Distribution of the DEC path type in cases and control individuals |
|||||
|
|
EAEC |
EPEC |
ETEC |
HYBRID |
DEC |
|
Diarrhoeic Children |
38,76 |
8 |
21,16 |
17,83 |
21,28 |
|
Non-diarrhoeic Children |
31,28 |
4,06 |
14,12 |
5 |
46,49 |
Enterotoxigenic Escherichia coli (ETEC)
In underdeveloped nations, the primary cause of vomiting in tourists and children is the E. coli pathotype ETEC that releases toxic substances in the human digestive tract. Children under the age of two have the greatest incidence of ETEC infection and death. The World Health Organization says that over 158 000 people have died from ETEC. instances of fatal diarrhea each year. ETEC infection is mostly characterized by moderate to severe diarrhea and stomach discomfort. There have very seldom been reports of additional complaints such vomiting, nausea, fever, and headache. Additionally, ETEC are animal infections that cause acute diarrheal illnesses in livestock, poultry, and piglets. In overall, colonisation determinants and contaminants are the two primary ETEC diseases that cause diarrhoea in people.
ETEC secretes enterotoxins after adhering to gastrointestinal epidermal cells through surface features. In the earliest stage of the pathway of disease of ETEC, colonization is crucial. By using several colonization factor (CFs) genes produced via plasmids that encode fimbrial, non-fimbrial, and fibrillar structures, ETEC interacts with tiny intestinal epithelial cells. CFA/I, CFA/II, and CFA/IV are three of the numerous CFs that are the primary mediators of ETEC colonisation and have been extensively found in ETEC isolated from clinical samples. E. coli common pili (ecpA), type 1 fimbriae (fimH), coli surface antigens (CS) like CS1-CS6, the Etp peptide family, and EatA (a member of the SAPTE family) are examples of various protein-based constructions, both fimbriated and non-fimbriated. ETEC's early adhesion, adhesin, and colonisation of the host intestinal epithelial cells are all facilitated by propellers. The type of glycoprotein EtpA released by ETEC contains N-acetylgalactosamine (NAG). NAG may also be seen as the glucose component on carbohydrates of the blood type A. Consequently, in those with Type A blood, ETEC makes dehydration sickness worse.
Enterohaemorrhagic Escherichia coli (EHEC)
EHEC are linked to multiple foodborne outbreaks in industrialized nations and are known to induce hemolytic uremic syndrome (HUS), hemorrhagic colitis (HC), and constipation in humans. The digestive system of cattle serves as this pathogen's primary reservoir.
In 1982, EHEC was initially discovered in a patient with bloody diarrhea and a digestive condition, which sparked an epidemic all over the globe. Individuals are mostly exposed to EHEC as foodborne A/E pathogens via tainted diets and drinking water. EHEC serotype incidents typically spread through the fecal-oral route through human-to-human contact, animal contact, and consuming unprocessed fruit juice with unprocessed cream, uncooked meat, or connect-contaminated uncooked produce like spinach and legume seeds. Additionally, newly discovered sources of EHEC epidemics include unclean wheat.
The most significant serotype implicated O157: H7, which is still prevalent in EHEC epidemics regarded as a substantial health threat in Americas, the European Union, and Japanese. Asparagus were consumed in Germany in 2011 resulted in the isolation of EHEC serotype O104: H4 from a disease of the digestive system and an epidemic of HUS. It then expanded all across the globe from there. However, this adenovirus's gene sequence showed that it is a member of both the enteroaggregative E. coli (EAEC) and enteroaggregative hemorrhagic E. coli (EAHEC) pathotypes, creating a novel E. coli pathotype.
The shiga-like toxin (SLT), also known as transcription and verotoxin by the stx genetics, is the primary pathogenicity component of EHEC variants that belong to the shiga-toxin generating E. coli group. It is in charge of the abnormalities that result in certain symptoms of sickness brought on by EHEC infections, such HUS and dialysis. Stx1 and Stx2 are two of the many subtypes that make up SLT. EHEC isolates that are positive for Stx2a, Stx2c, and Stx2d are significantly associated to HC and HUS when weighed against similar offering-subgroups and genotypes. An AB5 toxin is the shiga toxin. By weakening a disulfide link, subunit A is split into two pieces, A1 and A2, with the A1 fragment being released into the cytoplasm. It causes cell death and protein production to be inhibited by by enzymes deadenylating the 28s rRNA of the 60s ribosome at nucleotide 4324. The A1 segment of stx also stimulates produces cytokines and opens up avenues that lead to apoptotic also results in dying cells to inducing ribotoxic stress. The pathogenesis of shiga toxin subunit A is primarily caused by Immune reactions and the suppression of translated proteins regulation. To penetrate the target cell and provide a subunit segments must bind non-covalently with homopentameric B subunits to provide the damaging impact. The glycosphingolipid globotriaosylceramide (Gb3 or CD77), a particular receptor molecule present on the surface of human kidney epithelial and intestinal Paneth cells, is uniquely recognized by the B subunits of shiga toxins. Following receptors engagement and Gb3-stx complex formation, the associated poisons cluster together at the cell's plasma membrane, invaginate the membrane, and create endocytic pits. These invaginations create internal poison transporters by separating from the blood vessel membranes. Before stx escapes from the Gb3-stx complex to execute the cytotoxic impact, the endocytic transporters go throughout the organism, beginning with the early endoscopic and moving on to the Galleria and the endoplasmic reticulum. Through Gb3-independent transcytosis, micropinocytosis, and neutrophil transmigration, Stx toxins are delivered to non-target (Gb3-negative) cells. Stx toxins only cause a response of inflammation and do not stop the creation of proteins within Gb3-negative cells. The release of STX toxins by EHEC serotypes does not have a well-defined secretion mechanism. Shiga toxins are produced during phage-mediated lysis, and some causes, such as SOS-inducing substances like antibiotic treatment or DNA damage, suppress the translation of domains involved in the lytic phase. This aids in microbial cell disintegration of the spread of STEC and the shiga poison throughout surroundings.
A subset of STEC bacteria known as EHEC serotypes has Virulent components that encoding occurrence of the event and adherence are found on the LEE pandemic islands. EHEC colonizes abiotic and biological surfaces, forming biofilms, and this is facilitated by adhesin proteins.
Initial attachment is the first stage of EHEC adhesion to cells of the epithelium of the intestine. Contemporary research has shown that molecular components of the target cellular outer covering, such as fibronectin, collagen IV, and laminin, interact with the long polar fimbriae (lfp gene) of the pathogen to mediate the first pathogens and donor cell interaction and adhesion. Intimin, which is produced by the eae gene, interacts with the membrane-bound receptor proteins in the host cell, which include Tir (injected by T3SS), nuclein, and 1-integrins, to produce the A/E effect. In contrast to EPEC, EHEC has a distinct A/E pathogenic pathway. An Nck-independent mechanism mediates actin reorganization and pedestal creation. While the Tir-intimin complex binds to the EspF protein via a homologue protein of the insulin receptor substrate, cell actin cytoskeleton rearrangement is initiated. Through contact with the induced EspF, N-WASP and ARP2/3 are activated for actin assembly. The csg genes that encode curli are known to be adherence and colonization factors. E. coli common pilus (ECP) and hemorrhagic E. coli common pilus (HCP) engage contacting the target cellular interface to promote settlement, the creation of microcolonies, and the production of biofilms. The adherence to the host cell is facilitated by several proteins, including type 1, F9, and laminin-binding fimbriae from E. coli. EHEC releases proteins called autotransporters, such as Eha, Saa, and Sab, which help biofilms adhere. Additionally, the pO157 plasmid contains a gene called ToxB that may play a role in the adherence of serotype O157: H7 and other EHEC strains. It is important to remember that some environmental factors, such as pH, temperature, and nutritional deficiency, elicit the expulsion of supermajority-sensing particles, among other signalling methods, to amplify adhesin's production and transcribed proteins. In comparison to EPEC, Double the amount of receptors are secreted into the host cell by EHEC strains. through T3SS. The symptoms of GI illnesses are also influenced by colonization, the development of biofilms, and inflamed cellular reactions brought on by A/E. NleF, a non-LEE effector, is regarded as a significant due to the element of virulence to its ability to block cell death and suppress the Inflammation of the donor. Recent research has shown that a number of non-LEE-effectors, including NleB, NleE, BleG, NleH, and EspJ, regulate EHEC survivability and biofilm creation (figure 3).
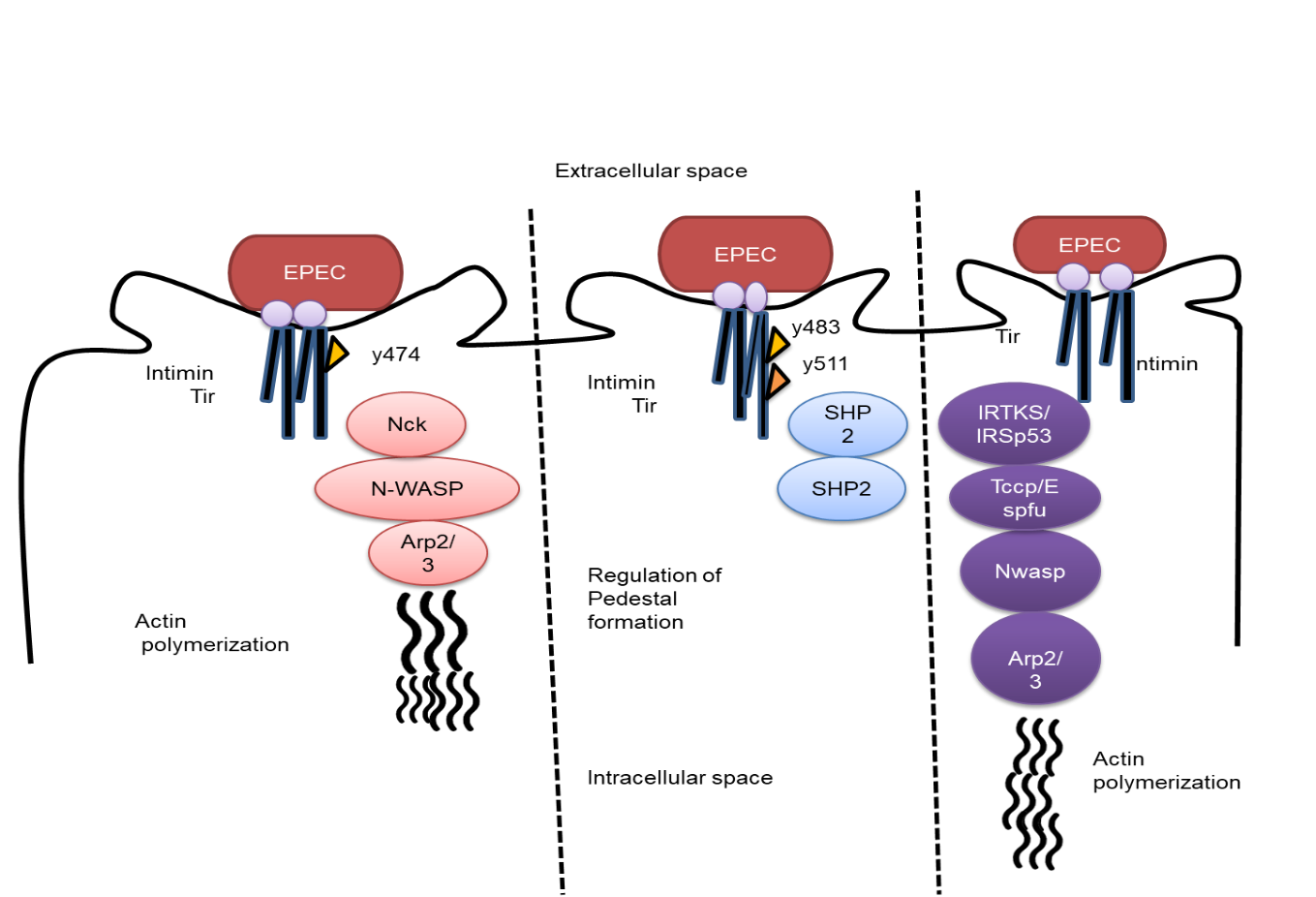
Figure 3. Escherichia coli that is enteropathogenic uses specific polymeric actin routes
Enteropathogenic Escherichia coli (EPEC)
The primary contributory factor of infantile intestinal infections and epidemics, which were first observed in the UK in 1945, is EPEC, which is characterized by a high prevalence of illness and mortality among infants below six months old. In wealthy nations, the EPEC rate has fallen in recent years. Yet, it is a significant issue with regard to healthcare for humans of all ages in underdeveloped nations. EPEC shares several pathogenic processes with diseases including E. coli, and Citrobacter rodentium in mice, rat-EPEC, and EHEC, such as the attachment and expelling (A/E) vectors. Items may transmit EPEC to both individuals and livestock and cause gastrointestinal illnesses, especially cream with meat from cattle.
According to histopathology, EPEC are A/E pathogens. The 35,62 kb chromosomal pathogenicity island (PAI), which contains an infectious plasmids named locus of enterocyte effacement (LEE) and EPEC adherence factor (EAF), together with 41 open-readable frames (ORFs), is where the majority of the virulence-factor-encoded genes of EPEC are found. The LEE-encoded regulator gene, which is found on the first segment of the LEE, controls the replication of the LEE gene. The EspC island is another PAI of EPEC that encodes the protein serine proteolytic autotransporter toxins genetic material, which is the sole toxin generated by EPEC that may cause epithelial cell necrosis. According to whether an EAF plasmid is present or not, EPEC is divided into two groups: typical (t-EPEC) with full gene expression and abnormal EPEC for bundle type IV pili lacking an operator (bfp) that harbors an EAF. The LEE is where the most noticeable EPEC virulence markers are found in both normal and atypical infections. This PAI is a type-three secretion system (T3SS), which includes regulating components, including the close adherence-related molecules Map, EspF, EspG, EspH, and EspZ, as well as the translocator molecules EspA, EspB, and EspD (EPEC secreted protein). Oxygen levels, hormones, and surroundings all have a role in the signaling of LEE-activating transcriptional. The proteins eae and tir that alternately encode for the TIM1 and the transferred TIM1 receptors are found on the membrane's outer layer. respond to start the A/E process. EPEC adheres to the surface of the host cells (initial connection) through pili-like proteins prior to T3SS expression after a stable connection by intimin-Tir interaction and the effectors injection. In the first stage of A/E after the development of localised microcolonies, type-IV pili (bfp) in a-EPEC and lymphoid inhibitory factor (lifA/efa1) in t-EPEC mediate the initial binding to the cells found in the tiny intestine.
T3SS is an intricate inject some equipment alongside more than 20 main amino acids that has its primary outside the transfer of the pore, hook, and filaments are examples of cellular attachments. It is financially made up of a cartridge with a major channel that is inserted into the outer layer of the by ring patterns and a supramolecular framework that is attached to the living cells on the highest of the thread. Different translocator structures of T3SS are built by the proteins espA, espb, and espd, which are then cleaved by espp and espc. A robust connection is created after Tir has been transferred Intimin and the transferred proteins engage in interaction. The creation of a pedestal in the target tissue underneath the pathogen attachment surface. and the stimulation of a cytoskeletal reorganization are the initial effects of the Tir-intimin connection. Tyrosine kinase in the host cell phosphorylates the moved Tir protein. When Tir is phosphorylated, the NcK protein is attracted, which activates Cell actin-related proteins (ARP) 2/3 is stimulated by the neural Wiskott-Aldrich syndrome protein (N-WASP), which influences the action of actin recruitment and reorganization that results in the formation of plinths. The immune system is influenced by this cytoskeletal reorganization, which also causes diarrhoea. The development of a dense coating is also essential to the pathophysiology of EPEC. Quorum-sensing, which is expressed by the suppressor of dividing inhibitory (SdiA) gene, activates the production of biofilms. Yet, in order to form biofilms, bacterium need to nourish these distinctive a substance called type filaments, and curli filaments that is produced by the EPEC proteins csgA, fimA, and bcsA, accordingly.
The T3SS facilitates the entry of LEE and NLE enzymes and receptors into the host cells is the next stage in EPEC pathogenesis. Many of these the effectors have the capacity to destroy the host cell. Tir suppresses NF-kB signaling Along with attachment and actin pedestal development as an immunity avoidance strategy in EPEC development. The Trp-xxx-Glu motif is shared by the mitochondrial-associated protein (MAP), which confers GTPase GEF activity. The Cdc42 factor is activated by GEF, which also aids in the development of filopodia into place at the bacterial growth point. MAP also impairs the operation of the nuclear membranes, which contributes to the demise of the host cell. NleA (espI), another well-known effector, performs a variety of functions, including rigid connection dislocation and NLRP3 inflammasome inactivation, and suppression of host cell cytokine release. EspF is a multi-functional effector that contributes to the immune evasion of EPEC throughout pathogenesis by triggering nuclear dying, compromising the integrity of tight junctions, and being involved in suction prevention. EspB, D, B2 and A genes also produced phagocytosis-inhibiting proteins. Another versatile non-LEE effector is nleF, which by attaching to caspase-4 stimulates the inflammatory system and suppresses the cellular immunological EspF, espT, MAP, nleF, and the cycle-inhibiting factor are the responses. (Cif) kill host cells by obstructing the cell cycle's development. One and many impacts of numerous Nle and esp molecules released by EPEC and other A/E infections remain to be defined, although the discovery of those signaling' roles.
Following group B2 (29/66, 43,9 %) and group D (6/66, 9,1 %), group A (31/66, 47 %) emerged as the dominant phylogenetic group among the DEC isolates. Group B1 was not found, however. In (figure 4), and the (table 3) the distribution of phylogenetic groupings among DEC types is shown.between DEC kinds and their distribution of phylogenetic groupings. Enteroaggregative E. coli (EAEC), tEPEC, typical enteropathogenic E. coli (aEPEC), and an average enteropathogenic E. coli.
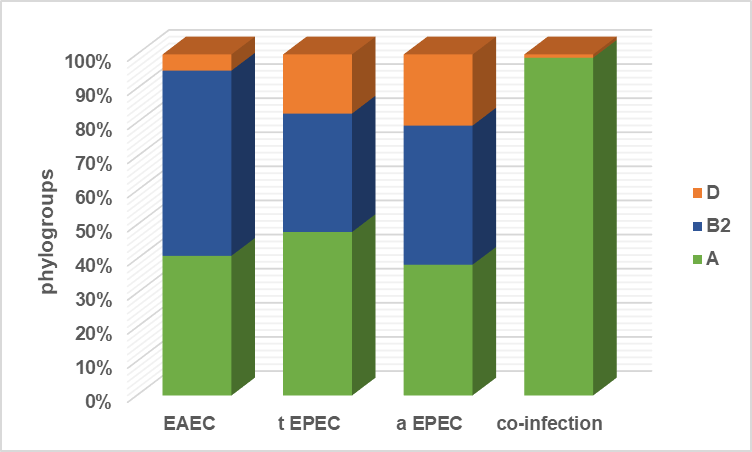
Figure 4. Typical enteropathogenic E.coli
|
Table 3. Common enteropathogenic E. coli |
|||
|
|
Phylogroups |
||
|
|
A |
B2 |
D |
|
EAEC |
43 |
57 |
5 |
|
t EPEC |
49,42 |
35,82 |
17,88 |
|
a EPEC |
39 |
41,49 |
21,23 |
|
co-infection |
100 |
0 |
1 |
CONCLUSIONS
There is a wide range of pathogenicity mechanisms and virulence factor profiles across enteric E. coli pathotypes. They still pose a serious threat to both food safety and human health. Through the adaptation of crucial genetic components, Different E. coli pathotypes that may produce poisons, colonize in groups, proliferate in the intestines, and affect different surroundings have emerged as a result of the development of colonic E. coli specific field. The development of novel E. coli pathotypes, such the organism of the genotype O104: H4 combination EAEC/STEC, resulted in fatalities all through Europe, highlights the need of a monitoring system. Such monitoring systems have to be integrated into one-health methods and networks because enteric pathogenic E. coli may spread and be contaminated via food, water, animals, and humans. Enteric E. coli pathotypes have an impact on a wide range of processes in epithelium digestive and extraintestinal host cells, such as synthesizing proteins, particular expression of genes, the release of various enterprises/ large molecules, particles, microtubule remodeling, autophagy, or metabolic processes, cell division, and signal transmission. A wide range of factors affecting virulence are involved, all of which are encoded by distinct chromosomal clusters of genes. Or cellular genetic components. Therefore, in order to collect exact data regarding pathogenic characteristics of several E. coli specific field for an appropriate monitoring, new methods like genome sequencing are needed. Additionally, a thorough examination of E. coli pathotype virulence genes' function in the evolution will aid in the creation of novel treatments, the enhancement of the design of efficient immunizations and the avoidance of additional propagation of such numerous and pervasive infectious agents.
REFERENCE
1. Pakbin B, Brück WM, Rossen JW. Virulence factors of enteric pathogenic Escherichia coli: A review. Int J Mol Sci. 2021;22(18):9922.
2. Kaper JB. Enterohemorrhagic Escherichia coli. Curr Opin Microbiol. 2020;1(1):103-108.
3. Fatima R, Aziz M. Enterohemorrhagic Escherichia Coli (EHEC). StatPearls [Internet]. 2020.
4. Yang SY, Yoon KS. Quantitative Microbial Risk Assessment of Listeria monocytogenes and Enterohemorrhagic Escherichia coli in Yogurt. Foods. 2022;11(7):971.
5. Cho SH, Lee KM, Kim CH, Kim SS. Construction of a lectin–glycan interaction network from enterohemorrhagic Escherichia coli strains by multi-omics analysis. Int J Mol Sci. 2020;21(8):2681.
6. Huerta-Saquero A, Chapartegui-González I, Bowser S, Khakhum N, Stockton JL, Torres AG. P22-Based Nanovaccines against Enterohemorrhagic Escherichia coli. Microbiol Spectrum. 2023;e04734-22.
7. Aditya A, Rahaman SO, Biswas D. Impact of Lactobacillus-originated metabolites on enterohemorrhagic E. coli in rumen fluid. FEMS Microbiol Ecol. 2022;98(12):fiac128.
8. Desvaux M, Dalmasso G, Beyrouthy R, Barnich N, Delmas J, Bonnet R. Pathogenicity factors of genomic islands in intestinal and extraintestinal Escherichia coli. Front Microbiol. 2020;11:2065.
9. Yekani M, Baghi HB, Naghili B, Vahed SZ, Sóki J, Memar MY. To resist and persist: Important factors in the pathogenesis of Bacteroides fragilis. Microb Pathog. 2020;149:104506.
10. Saeedi M, Khezri K, Seyed Zakaryaei A, Mohammadamini H. A comprehensive review of the therapeutic potential of α‐arbutin. Phytother Res. 2021;35(8):4136-4154.
11. Tosif MM, Najda A, Bains A, Kaushik R, Dhull SB, Chawla P, et al. A comprehensive review on plant-derived mucilage: Characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers. 2021;13(7):1066.
12. Kathayat D, Lokesh D, Ranjit S, Rajashekara G. Avian pathogenic Escherichia coli (APEC): an overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens. 2021;10(4):467.
13. Schuetz AN. Emerging agents of gastroenteritis: Aeromonas, Plesiomonas, and the diarrheagenic pathotypes of Escherichia coli. Semin Diagn Pathol. 2019;36(3):187-192.
14. Kim JS, Lee MS, Kim JH. Recent updates on outbreaks of Shiga toxin-producing Escherichia coli and its potential reservoirs. Front Cell Infect Microbiol. 2020;10:273.
15. Wang Y, Fu K. Genotoxins: The Mechanistic Links between Escherichia coli and Colorectal Cancer. Cancers. 2023;15(4):1152.
16. Amaretti A, Righini L, Candeliere F, Musmeci E, Bonvicini F, Gentilomi GA, et al. Antibiotic resistance, virulence factors, phenotyping, and genotyping of non-Escherichia coli Enterobacterales from the gut microbiota of healthy subjects. Int J Mol Sci. 2020;21(5):1847.
FINANCING
No financing.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: Asha Kademane, Meenal Dixit, Vasundhara.
Research: Asha Kademane, Meenal Dixit, Vasundhara.
Writing - proofreading and editing: Asha Kademane, Meenal Dixit, Vasundhara.