doi: org/10.56294/saludcyt2023452
REVIEW
The Influence of Developments in Tissue Engineering and Regenerative Medicine on Healthcare Advancement and Evolution
Influencia de los avances en ingeniería de tejidos y medicina regenerativa en el progreso y la evolución de la salud
Geetika Madan Patel1 ![]() *,
Nayana Borah2
*,
Nayana Borah2 ![]() *,
Gaurav Kumar3
*,
Gaurav Kumar3 ![]() *
*
1Parul University, Department of Community Medicine. PO Limda, Tal. Waghodia, Gujarat, India.
2JAIN (Deemed-to-be University), Department of Life Sciences. Karnataka, India.
3Teerthanker Mahaveer University, College of Nursing. Moradabad, Uttar Pradesh, India.
Cite as: Madan Patel G, Borah N, Kumar G. The Influence of Developments in Tissue Engineering and Regenerative Medicine on Healthcare Advancement and Evolution. Salud, Ciencia y Tecnología. 2023;3(S1):452. https://doi.org/10.56294/saludcyt2023452
Submitted: 08-06-2023 Revised: 30-06-2023 Accepted: 01-08-2023 Published: 02-08-2023
Editor: Dr.
William Castillo-González![]()
Associate Editor:
Fasi Ahamad Shaik ![]()
ABSTRACT
The area of regenerative therapy will undergo a revolution thanks to 3D bioprinting, which holds enormous potential for the bioprinting of artificial tissue and organs. The present research explores the potential synergies between 3D bioprinting and current developments in tissue engineering and regenerative medicine. Before 3D bioprinting is extensively used in organotypic structures for regenerative medicine, a number of obstacles must be solved. This places a significant burden on society in terms of providing care for those who have deteriorating organs and debilitating diseases. Researchers and medical experts are developing medications and technology that can repair tissues and even generate fresh ones in order to solve this problem. Tissue engineering and regenerative medicine strive to create new tissues and organs while also curing damaged or sick ones by fusing technology and biological principles. substantial breakthroughs in these domains have a substantial influence on 3D bioprinting of tissues and organs. The area of regenerative medicine might undergo a radical transformation thanks to the use of 3D bioprinting, which makes it possible to build new tissues and organs. The relationship between recent developments in tissue engineering, 3D bioprinting, and regenerative medicine is investigated in this paper. Before 3D bioprinting can be widely used to produce organotypic structures for regenerative medicine, a number of problems must be overcome.
Keywords: Tissue Engineering; Regenerative Medicine; 3D Bioprinting; Tissue and Organ 3D Bioprinting; Biomaterials and Cell Interactions.
RESUMEN
El área de la terapia regenerativa experimentará una revolución gracias a la bioimpresión 3D, que encierra un enorme potencial para la bioimpresión de tejidos y órganos artificiales. La presente investigación explora las posibles sinergias entre la bioimpresión 3D y los avances actuales en ingeniería de tejidos y medicina regenerativa. Antes de que la bioimpresión 3D se utilice de forma generalizada en estructuras organotípicas para la medicina regenerativa, hay que resolver una serie de obstáculos. Esto supone una importante carga para la sociedad en lo que se refiere a la atención a las personas con órganos deteriorados y enfermedades debilitantes. Los investigadores y expertos médicos están desarrollando medicamentos y tecnología capaces de reparar tejidos e incluso generar otros nuevos para resolver este problema. La ingeniería de tejidos y la medicina regenerativa se esfuerzan por crear nuevos tejidos y órganos y, al mismo tiempo, curar los dañados o enfermos mediante la fusión de la tecnología y los principios biológicos. Los avances sustanciales en estos campos tienen una influencia sustancial en la bioimpresión 3D de tejidos y órganos. El ámbito de la medicina regenerativa podría experimentar una transformación radical gracias al uso de la bioimpresión 3D, que permite construir nuevos tejidos y órganos. En este artículo se investiga la relación entre los últimos avances en ingeniería de tejidos, bioimpresión 3D y medicina regenerativa. Antes de que la bioimpresión 3D pueda utilizarse ampliamente para producir estructuras organotípicas destinadas a la medicina regenerativa, es preciso superar una serie de problemas.
Palabras clave: Ingeniería de Tejidos; Medicina Regenerativa; Bioimpresión 3D; Bioimpresión 3D de Tejidos y Órganos; Biomateriales e Interacciones Celulares.
INTRODUCTION
To repair, replace, or enhance the function of injured organs and tissues is the goal of tissue engineering, which necessitates significant attempts to integrate engineering, physical sciences, and biological sciences. Medical professionals have a major difficulty when treating organs and tissues that are diseased or damaged. Up until the latter half of the 20th century, patients with organ failure had limited alternatives for treatment.(1) Despite a continuing lack of donors, researchers from all over the world are frantically trying to find answers to the rising demand for organs. To address this widespread issue, scientists and engineers have created and integrated highly biocompatible yet delicate materials for tissue regeneration during the past 20 years.(2) Hydrogels are a particular class of biocompatible 3D polymers that may serve as a scaffold and imitate the characteristics of different bodily tissues. Tissue engineering and regenerative medicine cannot proceed without first defining and creating an acceptable material for tissue engineering. Because of their biocompatibility and structural closeness to the parts that make up the extracellular matrix, natural polymers have garnered a lot of interest over the past few years.(3)
Skin, heart, kidney, and liver are just some of the tissues and organs regenerative medicine has shown promise in regenerating and replacing, and it may even be possible to cure specific congenital abnormalities. Transplants traditionally rely on donated tissues and organs, which presents challenges due to donor limitations and the risk of immunological refusal.(4) It's also possible that the rate at which new tissue forms and is vascularized might increase. Microporosity is crucial for encouraging cell attachment and spreading, as well as for creating mechanical strength between the tissue and the scaffold in the first phases of healing. It is also critical to consider issues including degradation, biocompatibility, safety, stability, and cost-effectiveness in a clinical environment.(5) TERM wants to create an approach that can increase tissue regeneration by delivering growth hormones and increasing the mechanical qualities of the scaffold, therefore giving the cells a more favorable setting in which to differentiate. With this obstacle in mind, scientists and engineers work to offer cutting-edge ideas that will transform this sector of medicine.(6) They eventually deteriorate to leave only healthy tissue by integrating cells into their structure. They have gained a lot of popularity in recent years due to their ability to maintain a high water content, maintain a porous structure, and adjust to various sol-gel conditions.(7)
The strategy was developed using knowledge and data from pharmacogenetic and pharmacogenomic research. Sometimes, the phrases personalized medicine and precision medicine are synonymously.(8) The focus has shifted from "personalized medicine" to "precision medicine," nevertheless. Although pharmacogenetics gave rise to personalized medicine, it today encompasses many areas of healthcare. Yadid et al.(9) addressed the shape-dependent properties of gold nanoparticles, their universal application in developing tunable nanocomposite scaffolds with enhanced mechanical and electrical characteristics for tissue engineering, and the utilization of gold nanoparticle-integrated platforms to support stem cell proliferation and distinction. Shin et al.(10) offered multiple techniques for depositing CaP onto biomaterial surfaces and characterized the microstructure and natural bone mineralization process. They specifically outlined the benefits and development of biome metic surface-mineralizing techniques that use simulated bodily fluids to coat surfaces such as metals, ceramics, and polymers with carbonated apatite that resembles bone. Xu et al.(11) initially describes the characteristics of alginate and several techniques for creating alginate microgels. Then, they concentrated on various uses for alginate microgels in tissue engineering and regenerative medicine. Liu et al.(12) included current developments in biomimetic natural biomaterials (BNBMs), including their functioning, preparation, possible uses, and upcoming difficulties. To advance this perspective medical strategy toward future clinical application, Liu et al.(13) focused on the existing knowledge and prospects surrounding accessible bio-inks, bioprinting techniques, and the most recent advancements in cardiac 3D Bioprinting.
Faramarzi et al.(14) described the creation of a bio-ink based on alginate that may be printed and crosslinked after implantation by being exposed to natural calcium ions. This technology may be utilized to deliver growth factors related to PRP in a regulated manner, perhaps enhancing vascularization and stem cell migration. Pang et al.(15) summarised the pathophysiology of wound healing and examined the most recent findings on leveraging the mainstays of the field of regenerative medicine (growth hormones, stem cell treatment, biological materials, and tissue engineering) as goals for therapy.
This paper examines how regenerative medicine, tissue engineering, and 3D bioprinting work in concert while emphasizing developments in bioprinting technology for transplantable organs and tissues. The emphasis is on mechanical properties, exact mechanical replication of human organs and tissues, and particular applications in the tissue engineering of cartilage, the heart, and the liver.
METHODS
Although we largely concentrated on the latter years to give the most recent technical advancements, a literature search for the following key terms was conducted from 2005 to 2022 using the databases Science Direct, Google Scholar, and PubMed. Stem cells, tissue engineering, organ transplantation, regenerative medicine, native tissue, 3D Bioprinting, biologics, and biomaterials.
RESULTS
Substitution of human organs and tissues
Organs and tissues made through tissue engineering are intended to closely mimic their natural counterparts. Using decellularization and recellularization techniques is one effective way. Decellularization eliminates cells from a tissue or organ, leaving the acellular extracellular matrix (ECM) in its place. This decellularized ECM can be employed as a bio-ink in 3D Bioprinting or as a scaffold for tissue regeneration. The capacity to mimic tissue-specific characteristics and supply vital signals for cell development and differentiation are only two advantages provided by decellularized ECM.(16) Decellularizing detergents can change the ECM's mechanical features, though. Recellularization, which entails introducing cells to the decellularized ECM, helps to overcome this. Different cell types may be created from stem cells, frequently called differentiated cells. The appropriate circumstances can allow stem cells to adhere to the ECM and aid in tissue regeneration.
Decellularization has produced positive results in animal models, making employing acellular tissues and organs as therapeutic devices possible without the requirement for recellularization. This eliminates the laborious recellularization procedure, which can hasten the release of acellular products. However, synthetic scaffolds built from ECM proteins and synthetic polymers cannot wholly replicate the intricate features of actual tissues and organs. The use of hydrogels in tissue engineering is fascinating since they naturally disintegrate and mimic genuine tissues. In order to cure congenital heart defects, they can act as scaffolds for the construction of vascular grafts.(17) Tissue engineering and the area of regenerative medicine can both benefit from composite scaffolds comprised of natural and synthetic biomaterials by offering cell-recognition sites for adhesion and proliferation.
Although the specific function of seeded cells in tissue formation is not entirely known, they aid in the inflammation required for graft integration and the host cells' production of new blood vessels. It may be beneficial to instruct implanted cells to create tissue-specific ECM proteins. Depending on the unique physical characteristics of the patient, customized replacement tissues can be made utilizing noninvasive imaging techniques like computed tomography (CT) and magnetic resonance imaging (MRI). For instance, these techniques have been used to create polymer-based, patient-specific scaffolds for organs such as the trachea. To build functioning tissues and organs, tissue engineering uses techniques including decellularization and recellularization. Due to their tissue-specific properties and biodegradability, hydrogels and decellularized ECM are suitable scaffold materials. The introduction of organic and synthetic biomaterials further improves the performance of scaffolds. We are getting closer to using seeded cells and noninvasive imaging technologies to create patient-specific replacement tissues for use in various medicinal applications.
3d bioprinting
The capacity to precisely control the placement of cells within scaffolds, which has revolutionized the use of platforms and cells together, is a significant advantage of 3D Bioprinting. Currently, inkjet, microextrusion, and laser-assisted printing techniques are used in 3D bioprinting. By using cells that can produce ECM proteins like collagen and fibronectin, these techniques have been effective in producing a variety of tissues, including cartilage, aortic valves, and blood vessels. Despite the development of several bioprinting processes, challenges still exist.(18) The low cell viability during 3D Bioprinting is one of the main difficulties. A graphene-polyurethane nanocomposite hydrogel, however, has recently demonstrated potential as a bio-ink for printing cell-rich tissues. While retaining its shear-thinning qualities, this hydrogel has shown benefits for regenerating brain tissue. The absence of circulatory tissues in 3D bioprinted tissues is a serious flaw that can cause cell death from a lack of nutrients and oxygen. Researchers have developed a method to solve this problem that enables the 3D printing of perfused vascular channels by covering a network of carbohydrate glass filaments with extracellular matrix and endothelial cells. These channels have helped cellular metabolism, the manufacture of drug transporter proteins, and the production of metabolic enzymes in bioprinted liver designs.
The properties of graphene-based nanoparticles include large surface area, chemical stability, and the potential to promote stem cell proliferation and differentiation in tissue engineering and regenerative medicine. One needs a good grasp of the chemistry and spatial arrangement of tissue components to faithfully replicate the intricate architecture of tissues and organs. Noninvasive imaging methods including MRI, CT, and computer-aided design (CAD) are used to create 3D models of tissues and organs. These models may be further improved by applying mathematical modeling software and CAM-CAD procedures to forecast critical mechanical and biological properties. In tissue engineering, 3D Bioprinting enables precise material and cell positioning. To address problems with vascularization and cell survival, novel strategies are being explored. Thanks to graphene-based materials and noninvasive imaging methods, tissue engineering has evolved. The emergence of bioprinting techniques offers significant potential for tissue engineering, even though not all printing techniques are discussed in this paper.
Bioprinting using inkjet
Inkjet printers, often drop-on-demand printers, have nonbiological and biological uses. In essence, inkjet paper printers readily accessible on the market were transformed into natural material printers. Liquid biological material is sprayed in large volumes with excellent resolution, accuracy, and speed onto designated surfaces. Liquids are discharged from the printer onto a substrate or scaffold using thermal or acoustic pressures. The substrate or scaffold is frequently a part of the graft that will be implanted into the tissue.
Heat-activated print heads that release biological material onto the scaffold as droplets are used in thermal inkjet printers.(19) The process of heating has no impact on the integrity or quality of the natural material. The least expensive and most often used of the three bioprinting techniques is the thermal inkjet printer. Inkjet printers may be utilized with a variety of biological materials. In acoustic printers, piezoelectric crystals generate sound waves. Changing the wave's amplitude and duration in the printer head may alter the natural material droplet's size. Using acoustic inkjet printers, it is relatively simple to regulate the biological material droplet's scope and direction of ejection. One drawback of inkjet printers is that the printed natural material must be kept at a specified viscosity. Whenever a thickness is exceeded, the printer head may clog. The number of cells put into and afterward printed is frequently decreased to maintain biological components in a liquid state. High cell concentrations put droplet generation at risk and make it more likely that the printer head may clog.
Microextrusionbioprinting
Microextrusion is a commonly utilized method in the study of tissue and organ engineering. It entails depositing biological material onto a scaffold or substrate using a robot equipped with a microextrusion head. Extrusions of natural material, such as hydrogels or cellular spheroids, are continually carried out onto the scaffold under the guidance of CAM-CAD technology. Both mechanical and pneumatic extrusion techniques are frequently used in microextrusion; the automated approach dispenses the biological material using a screw or piston, while the pneumatic system uses compressed air.(20) Biological materials with a range of viscosities may be printed using microextrusion technology.
The ability to print with high cell densities using microextrusion technology makes it possible to create tissues with densities of cells similar to those found in physiological environments. Microextrusion may also be used to print cellular spheroids, capable of self-assembling into 3D objects. Scientists think that cellular spheroids and tissue extracellular matrix (ECM) have similarities, making it possible to create vascular tissue spheroids that self-assemble into 3D bio-printed organs. Lower cell survival relative to inkjet printing is a disadvantage of microextrusion bioprinting. Despite this drawback, microextrusion has been utilized to effectively produce a variety of tissues, including heart valves, vascular networks, and tumor models.
Bioprinting using laser technology
Laser-assisted Among other biological elements, bioprinting has been used to print peptides, cells, and DNA. This method is less common than inkjet and microextrusion bioprinting. This method(21) blasts pressurized bubbles into the scaffold or substrate using laser pulses to produce pressured bubbles. (Figure 1). There is no printer head clogging because there is no nozzle involved in this operation. Additionally, the method functions with a range of viscosities. This shows that cellular viability and function can be preserved despite achieving cell densities comparable to those in physiological tissue. The method's main disadvantage is the presence of metallic residues in the final printed product owing to metallic residue formation during printing. Although the cost of using this technology is likewise relatively high, it is anticipated to fall with time. Laser-assisted Bioprinting has been used to create a number of animal and human tissues.
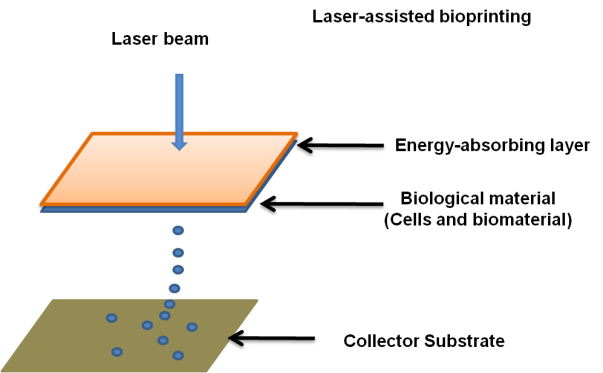
Figure 1. Laser-assisted bioprinting
Contemporary aspects of tissue engineering and regenerative medicine
Making functioning tissues and transplants requires careful consideration when choosing biomaterials and biological sources. Cells and biomaterials must interact for the scaffold to work at its best.(22) Normal tissue should have a similar range of cell types and functions as regenerated tissue. For instance, endothelial cells are crucial for the structural stability and effective operation of 3D bioprinted grafts or scaffolds, which substantially impacts construct performance. For successful integration and long-term survival, transplanted grafts should be able to replace themselves and maintain homeostasis. Autologous patient cells are excellent because they reduce immune reactions. Before 3D Bioprinting or transplanting, these cells can be grown in vitro and instructed to develop into the required cell types. However, autologous cells have limited regenerative potential in early-stage cells and restrictions in cell culture. The level of control offered by 3D Bioprinting is higher than that of acellular printing methods. The functionality of transplants within the body depends on their successful fusion with patient vasculature. The proximity of blood arteries to cells enables oxygen exchange and nutrition delivery. Essential neurons and blood vessels are frequently challenging to generate using conventional techniques. However, the creation of vessels in designed tissues has been aided by angiogenic growth factors, including VEGF, bFGF, and PDGF. They pose challenges because of their short half-lives and related detrimental effects.
Growth factors released continuously can prevent tissue necrosis. Another strategy to encourage graft vascularization is revascularization, accomplished by inserting endothelial cells into the suitable material during 3D Bioprinting and subsequent implantation. Methods being researched for forming or activating tissue vascular networks include microfluidics and micropatterning. If tissue engineering and regenerative medicine are to advance, biomaterials and biological sources must be carefully considered. Despite their disadvantages, autologous cells are preferred to prevent immunological reactions. While 3D Bioprinting offers better control and integration choices, revascularization procedures give alternatives for improving graft vascularization.(23) Although revascularization enhances the integration of transplanted grafts, innervation is still necessary for some tissues to operate at their best. To promote the proliferation of neurons in transplanted tissues, hydrogels containing channels conveying growth agents and extracellular matrix (ECM) proteins can be made.
To be chosen for 3D Bioprinting, cells must be able to differentiate, endure printing, be resilient, and have the capacity to grow. They should interact through direct contact or the release of biomolecules like cytokines and growth factors after transplantation, performing biological tasks similar to those of native cells. Because they can self-renew and differentiate, adult and embryonic stem cells are favored for 3D Bioprinting. Adult stem cells are expected to be safe for transplantation and functioning after printing. The host tissue responds to the introduction of foreign cells by producing growth factors. Transplanted cells can alter the extracellular matrix (ECM) of the recipient by secreting growth hormones, ECM-degrading enzymes, or new ECM proteins. Even without direct interaction between the transplanted and host cells, a therapeutic response may still take place. Mesenchymal stem cells (MSCs) are assumed to be more easily accessible from adult tissues and less harmful than embryonic cells, which makes them highly beneficial for tissue regeneration.
Adult tissue-derived cells are used to create a variety of commercially available therapies. In regenerative medicine, both embryonic stem cells and induced pluripotent stem cells (iPSCs) can be used. Clinical studies have demonstrated the safety of embryonic stem cells, while iPSCs created from a patient's own cells ease concerns about rejection. Therapeutic value from transplanted cells on a scaffold is minimal since the host tissue soon destroys them.(24) The danger of rejection is decreased when cells are enclosed in substances like hydrogels. Cells may be directed to particular organs and tissues using peptides and antibodies. Technological developments can change the qualities of the scaffold to reduce graft rejection, boost graft tolerance, and actively encourage tissue regeneration and engraftment.
The effects of biomaterials and cell interactions on 3d bioprinting
It is essential to look at how cells interact with biomaterials in order for their usage in tissue engineering to be successful. By simply changing their mechanical properties by crosslinking, synthetic polymers have the benefit of providing control over cellular microenvironments. In tissue engineering, hydrogels are frequently utilized because they can replicate the properties of soft tissues. In order to encourage cellular growth and differentiation, they can contain biomolecules like fibronectin, collagen, and matrige.(25) Hydrogels frequently contain natural polymers, including collagen, gelatin, fibrin, and chitosan, yet these materials might trigger immune reactions. Custom synthetic polymers are also created for certain tissues or organs. Since natural polymers can communicate biological signals for cell development and differentiation, they are favored for 3D Bioprinting. Hyaluronic acid-based hydrogels have been used to treat joints, although they may have side effects such as edoema and softness.
Despite its weak structure and high price, collagen is still employed in 3D Bioprinting. By adding biological signals for tissue regeneration, biomaterials may be created to progressively deteriorate and be replaced by new tissue over time. To establish the biocompatibility and efficacy of biomaterials, in vitro testing, composition development, and in vivo assessments are crucial procedures.Results from clinical trials are crucial for optimizing biomaterial design and comprehending treatment procedures.(26) Scaffolds can be made more bioactive by including growth factors, enzyme recognition sites, and adhesion molecules. Particularly in the early stages of tissue regeneration, hydrogels allow for the regulated release of biomolecules and bioactive substances.
Biomaterial design tries to stimulate cell proliferation, adhesion, and immunological control for a transplant to be effective. The interaction of biomaterials and tissues can trigger cellular and immunological reactions. Specific properties are required for integration into host tissue. By maintaining biological activity and promoting graft-host signaling, biomaterials are essential to 3D Bioprinting. Tissue engineering requires careful biomaterial selection and design. Hydrogels and synthetic polymers provide control over cellular microenvironments and the inclusion of bioactive substances. Enhancing cellular adhesion, differentiation, and tissue regeneration are scaffolds that release growth factors. For tissue engineering to improve, biocompatibility, immunological responses, and interactions between biomaterials and cells must be carefully considered
.
Tissue 3d bioprinting
Regeneration of cartilage
Although cartilage is essential for painless mobility, accidents and diseases like osteoarthritis can cause it to degenerate. Effective cartilage healing requires the replication of both the stromal tissue and the cartilage surface. Implants made of synthetic metal and plastic have problems, such as accumulating foreign material and losing efficiency over time. The use of chondrocytes and MSCs in recent methods to regenerate cartilage has yielded mixed results since the mechanics of cartilage growth are still poorly understood. According to research, adipose-derived MSCs can control the chondrogenic lineage during cell growth.(27)
Hyaluronic acid enhances lubrication in biomaterials intended to promote the growth of new cartilage that mimics the stromal tissue of cartilage. When cells and biomaterials are used together, regeneration is better than individually. In translational research, many stem cell biomaterial assemblages are being studied. It has been discovered that strong, flexible materials can promote stem cell chondrogenesis. PEG hydrogels constructed of various polymers are being investigated for their potential to treat cartilage defects, and complex structures made of type II collagen, Magellan, and alginate have demonstrated enhanced chondrocyte proliferation and biocompatibility.(28)
Organs 3d bioprinting
The intricacy of genuine organs requires precise positioning of many cell types, making organ 3D bioprinting difficult. Blood, arteries, and nerves are also essential for an organ to operate. The challenge of mass-producing these intricate organs for in vivo transplantation still has to be resolved larger; more complex tissues and organs are still challenging to print, and cell viability may be impaired. Adding biomolecules like chemokines and growth factors can increase the viability of cells both before and after Bioprinting.(29) Bioreactors have transformed the post-printing process by offering the appropriate setting for long-term culture or preservation of the printed scaffold or graft. Bioreactors mimic the frequent exchanges of nutrients, oxygen, and biomolecules observed in healthy organs to promote the scaffold or graft's long-term development. To integrate into host tissue, cells, extracellular matrix (ECM), and cell surface receptors must all cohabit in harmony. Despite the difficulties, researchers and medical professionals are still attempting to develop the ability to print organs, and the development of mini-organs may represent a future development in this area.
Heart
The heart, one of the earliest organs to form, plays a critical role in the body's ability to circulate blood. It is a complicated and muscular organ with cardiomyocytes, endothelial cells, and fibroblasts. With the lack of organ donors, 3D Bioprinting has become a promising cure for heart failure. Bioprinting technology is being investigated by scientists for the construction of cardiac structures and heart transplants. For the heart to operate at its best, proper vascularization and innervation are essential, which presents a problem for artificial cardiac structures and transplants. The extracellular matrix (ECM) of the heart, which is necessary for cellular differentiation and protein production, is mostly composed of collagen.(30) For cardiac tissue engineering, a number of methods, such as the use of allografts, xenografts, and autografts, have been investigated. Heart disease treatment and heart repair hold great promise for the future of tissue engineering and regenerative medicine. Through the use of 3D Bioprinting, functioning cardiac tissue and heart valves have been successfully produced. When printing heart valves, biodegradable polymers are frequently employed because they enable accurate reproduction of valvular shape. Different cell types and 3D bioprinting techniques were used to manufacture beating heart tissue.
To encourage the formation of blood arteries, patches have been printed with mesenchymal stem cells (MSCs) and endothelial cells. Gene expression studies have shown good cell survival and development into the cardiac lineage for the majority of 3D bioprinted cells. The use of engineered myocardial tissue as a possible replacement in cases of severe heart damage caused by conditions like coronary artery blockages or myocardial infarction appears promising. Improved cardiac tissue repair and cell survival have been shown with bioprinting techniques utilizing functionally tailored patches, such as an alginate hydrogel containing cardiomyocyte progenitor cells. Cardiac tissue has also been produced using decellularized heart tissue and microextrusion bioprinting.(31) Compared to nonliving prostheses, the functioning of live prosthetics has been improved via Bioprinting, allowing for greater heart condition adaptability and integration.
Liver
Hepatocytes, which make up the majority of the liver, carry out a variety of metabolic tasks, such as the creation of hormones, detoxification, and protein synthesis. Endothelial cells and portal fibroblasts are two more cell types seen in the liver. The incredible capacity for regeneration of hepatocytes helps the liver to recover from harm. Hepatocytes, however, rapidly lose their functionality when they are grown in vitro. The ideal cell source for 3D Bioprinting of liver tissue is adult stem cells taken from the patient since they may express genes that are comparable to hepatocytes.(32) The creation of microlivers for high-throughput testing of treatment possibilities is made possible as a result. In several cellular sources, liver tissue has been produced or transplanted using a variety of cell types, such as stromal cells generated from adipose tissue, Wharton jelly, and hepatic progenitor cells.
The printed cells secrete albumin and have characteristics similar to hepatocytes. The complexity of 3D bio-printed liver tissues has been considerably improved by the incorporation of endothelial cells. These liver structures exhibit liver-specific activity and injury response. Artificial livers that resemble the form and operation of actual organs have been created by a number of businesses and research groups. There is a great demand for livers. Thus, it would have a big impact if it were able to generate liver tissue or whole livers. Tissue engineering has a huge problem in replicating the intricate modular organization of natural liver tissue.
Medical treatments in tissue engineering and regenerative medicine
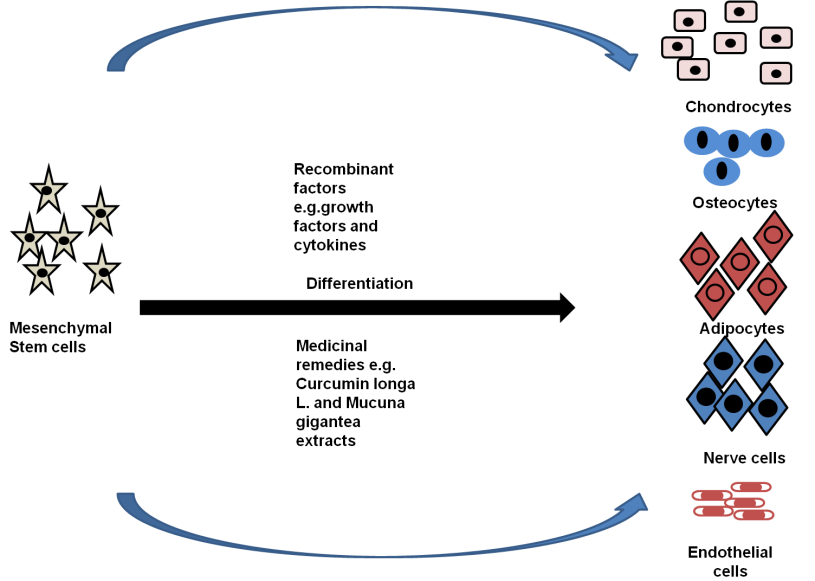
Concerns concerning toxicity and negative consequences have been expressed regarding the use of biological agents such as growth factors in stem cell treatments and 3D bioprinting procedures. Additionally, the fact that they come from nonhuman origins may reduce their effectiveness.(33) On the other hand, research is being done on the potential of herbal extracts for tissue regeneration, stem cell proliferation and differentiation, and tissue repair. (figure 2)
Figure 2. Artificial factors and medicines can differentiate mesenchymal stem cells into distinct cell types. Drugs are inexpensive and have little negative effects
The utilization of medicinal plant extracts in tissue regeneration and stem cell multiplication illustrates the possibility of affordable and efficient therapies. But there are still problems to be tackled, including a lack of comprehension of the underlying mechanisms, the complexity of the extract, toxicity, and predictability. Purification and standardization of these extracts are necessary before they can be used effectively in regenerative therapies. Despite these difficulties, medical plant extracts have benefits, including affordability, efficiency, and minimal toxicity. In combination medicines, their various phytochemicals may have additive or synergistic effects. To completely understand the mechanisms and signaling pathways underpinning their therapeutic potential, more study is required. Tissue engineering and stem cell treatments that use medicinal plant extracts can enhance the area of regenerative medicine and offer secure and affordable healthcare options. Notably, extracts from Mucuna gigantea have demonstrated higher performance in the formation of brain cells, radix angelica sinensis extract containing ferulic acid displays neuroprotective benefits, and salvia miltiorrhiza extract encourages neurogenic growth.(34) To avoid negative effects and guarantee appropriate utilization of these extracts from varied plant sources, careful selection of solvents and purification techniques is essential.
Challenges in tissue and organ 3d bioprinting
Due to its multidisciplinary character, 3D Bioprinting requires the cooperation of researchers from several domains in order to be successfully implemented. There are a few challenges to be resolved before going from proof-of-concept examples to actual tissue and organ bioprinting. It is challenging to create uniform methods for arranging and constructing tissues and organs due to the variations in cells gathered from different individuals and their unique patterns of proliferation and differentiation.(35) It is necessary to find solutions to problems like the lengthy bioprinting process and the biocompatibility of materials. Additionally, the integration of diverse bioprinting methods would be necessary to print numerous biomaterials and cell types simultaneously in particular scaffold portions. Another important factor is the maturity of the scaffold or structure in a bioreactor. Vascularization is a major barrier to tissue engineering and regenerative medicine, yet 3D Bioprinting provides workable answer.(36)
Successful vascularized 3D tissue production has been demonstrated in both animal and human experiments. Another benefit of 3D Bioprinting is its adaptability to individual demands. To guarantee that the finished construct or graft is suitable for human usage, high-quality Bioprinting is necessary. At every stage of the procedure, strict quality control that complies with pharmaceutical standards is required. Before structures and grafts may be used, regulatory agencies like the FDA or the European Medicines Agency must provide their clearance.(37) Despite current challenges, tissue engineering and regenerative medicine offer immense potential. To fully realize the potential of engineering approaches like Bioprinting, a collaboration between academics and practitioners is required.(38) The adaptability of 3D Bioprinting also makes it possible to produce organs and tissues for study in fields like cancer therapy and medication toxicity.
CONCLUSION
More and more illnesses and ailments are being treated with regenerative medicine. The ability to precisely manage the scaffolds and cells in 3D-bioprinted structures or organs has the potential to affect how the host reacts. Grafts can be manufactured specifically for a patient, placing cells where they are needed to imitate natural tissues. Enhancing graft integration with host tissue requires a better knowledge of graft vascularization and innervation. Healing and regeneration are encouraged by the carefully controlled release of growth factors within bioprinted constructions. Immunological system modification lowers rejection or produces desired immunological outcomes. The behavior and development of stem cells may be better understood, and their security can be guaranteed. Rejection can be avoided, and a good niche for transplanted cells can be created by altering the host environment. It is acknowledged that the microbiome plays a part in integration. For the translation of 3D-bio-printed illness models into clinical practice, improvements are required. Innovative biomaterials and technologies, such as nanotechnology that replicate and cooperate with nature in tissue engineering are essential to the future of regenerative medicine.
REFERENCES
1. Shafiee A, Atala A. Tissue engineering: toward a new era of medicine. Ann Rev Med. 2017;68:29-40. https://doi.org/10.1146/annurev-med-102715-092331
2. Mantha S, Pillai S, Khayambashi P, Upadhyay A, Zhang Y, Tao O, Pham HM, Tran SD. Smart hydrogels in tissue engineering and regenerative medicine. Materials. 2019;12(20):3323. https://doi.org/10.3390/ma12203323
3. Sultankulov B, Berillo D, Sultankulova K, Tokay T, Saparov A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules. 2019;9(9):470. https://doi.org/10.3390/biom9090470
4. Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, Pillay M, Motaung KSCM. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int. 2018;2018. https://doi.org/10.1155/2018/2495848
5. Pina S, Ribeiro VP, Marques CF, Maia FR, Silva TH, Reis RL, Oliveira JM. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials. 2019;12(11):1824. https://doi.org/10.3390/ma12111824
6. Vial S, Reis RL, Oliveira JM. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr Opin Solid State Mater Sci. 2017;21(2):92-112. https://doi.org/10.1016/j.cossms.2016.03.006
7. Deshmukh K, Kovářík T, Křenek T, Docheva D, Stich T, Pola J. Recent advances and future perspectives of sol–gel derived porous bioactive glasses: a review. RSC Adv. 2020;10(56):33782-33835. https://doi.org/10.1039/D0RA04287K
8. Arjmand B, Goodarzi P, Mohamadi-Jahani F, Falahzadeh K, Larijani B. Personalized regenerative medicine. Acta Med Iran. 2017:144-149.
9. Yadid M, Feiner R, Dvir T. Gold nanoparticle-integrated scaffolds for tissue engineering and regenerative medicine. Nano Lett. 2019;19(4):2198-2206. https://doi.org/10.1021/acs.nanolett.9b00472
10. Shin K, Acri T, Geary S, Salem AK. Biomimetic mineralization of biomaterials using simulated body fluids for bone tissue engineering and regenerative medicine. Tissue Eng Part A. 2017;23(19-20):1169-1180. https://doi.org/10.1089/ten.tea.2016.0556
11. Xu M, Qin M, Cheng Y, Niu X, Kong J, Zhang X, Huang D, Wang H. Alginate microgels as delivery vehicles for cell-based therapies in tissue engineering and regenerative medicine. Carbohydr Polym. 2021;266:118128. https://doi.org/10.1016/j.carbpol.2021.118128
12. Liu S, Yu JM, Gan YC, Qiu XZ, Gao ZC, Wang H, Chen SX, Xiong Y, Liu GH, Lin SE, McCarthy A. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications. Mil Med Res. 2023;10(1):1-30. https://doi.org/10.1186/s40779-023-00448-w
13. Liu N, Ye X, Yao B, Zhao M, Wu P, Liu G, Zhuang D, Jiang H, Chen X, He Y, Huang S. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact Mater. 2021;6(5):1388-1401. https://doi.org/10.1016/j.bioactmat.2020.10.021
14. Faramarzi N, Yazdi IK, Nabavinia M, Gemma A, Fanelli A, Caizzone A, Ptaszek LM, Sinha I, Khademhosseini A, Ruskin JN, Tamayol A. Patient‐specific bio-inks for 3D bioprinting of tissue engineering scaffolds. Adv Healthc Mater. 2018;7(11):1701347. https://doi.org/10.1002/adhm.201701347
15. Pang C, Ibrahim A, Bulstrode NW, Ferretti P. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int Wound J. 2017;14(3):450-459. https://doi.org/10.1111/iwj.12735
16. Savoji H, Godau B, Hassani MS, Akbari M. Skin tissue substitutes and biomaterial risk assessment and testing. Front Bioeng Biotechnol. 2018;6:86. https://doi.org/10.3389/fbioe.2018.00086
17. Goodarzi P, Falahzadeh K, Nematizadeh M, Farazandeh P, Payab M, Larijani B, Tayanloo Beik A, Arjmand B. Tissue-engineered skin substitutes. In: Cell Biology and Translational Medicine, Volume 3: Stem Cells, Biomaterials, and Tissue Engineering, pp.143-188. DOI: 10.1007/5584_2018_226
18. Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR. Bioinks for 3D bioprinting: an overview. Biomater Sci. 2018;6(5):915-946. https://doi.org/10.1039/C7BM00765E
19. Li X, Liu B, Pei B, Chen J, Zhou D, Peng J, Zhang X, Jia W, Xu T. Inkjet bioprinting of biomaterials. Chem Rev. 2020;120(19):10793-10833. https://doi.org/10.1021/acs.chemrev.0c00008
20. Shafiee A, Atala A. Tissue engineering: toward a new era of medicine. Ann Rev Med. 2017;68:29-40. https://doi.org/10.1146/annurev-med-102715-092331
21. Mantha S, Pillai S, Khayambashi P, Upadhyay A, Zhang Y, Tao O, Pham HM, Tran SD. Smart hydrogels in tissue engineering and regenerative medicine. Materials. 2019;12(20):3323. https://doi.org/10.3390/ma12203323
22. Sultankulov B, Berillo D, Sultankulova K, Tokay T, Saparov A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules. 2019;9(9):470. https://doi.org/10.3390/biom9090470
23. Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, Pillay M, Motaung KSCM. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int. 2018;2018. https://doi.org/10.1155/2018/2495848
24. Pina S, Ribeiro VP, Marques CF, Maia FR, Silva TH, Reis RL, Oliveira JM. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials. 2019;12(11):1824. https://doi.org/10.3390/ma12111824
25. Vial S, Reis RL, Oliveira JM. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr Opin Solid State Mater Sci. 2017;21(2):92-112. https://doi.org/10.1016/j.cossms.2016.03.006
26. Deshmukh K, Kovářík T, Křenek T, Docheva D, Stich T, Pola J. Recent advances and future perspectives of sol–gel derived porous bioactive glasses: a review. RSC Adv. 2020;10(56):33782-33835. https://doi.org/10.1039/D0RA04287K
27. Arjmand B, Goodarzi P, Mohamadi-Jahani F, Falahzadeh K, Larijani B. Personalized regenerative medicine. Acta Med Iran. 2017:144-149.
28. Yadid M, Feiner R, Dvir T. Gold nanoparticle-integrated scaffolds for tissue engineering and regenerative medicine. Nano Lett. 2019;19(4):2198-2206. https://doi.org/10.1021/acs.nanolett.9b00472
29. Shin K, Acri T, Geary S, Salem AK. Biomimetic mineralization of biomaterials using simulated body fluids for bone tissue engineering and regenerative medicine. Tissue Eng Part A. 2017;23(19-20):1169-1180. https://doi.org/10.1089/ten.tea.2016.0556
30. Xu M, Qin M, Cheng Y, Niu X, Kong J, Zhang X, Huang D, Wang H. Alginate microgels as delivery vehicles for cell-based therapies in tissue engineering and regenerative medicine. Carbohydr Polym. 2021;266:118128. https://doi.org/10.1016/j.carbpol.2021.118128
31. Liu S, Yu JM, Gan YC, Qiu XZ, Gao ZC, Wang H, Chen SX, Xiong Y, Liu GH, Lin SE, McCarthy A. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications. Mil Med Res. 2023;10(1):1-30. https://doi.org/10.1186/s40779-023-00448-w
32. Liu N, Ye X, Yao B, Zhao M, Wu P, Liu G, Zhuang D, Jiang H, Chen X, He Y, Huang S. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact Mater. 2021;6(5):1388-1401. https://doi.org/10.1016/j.bioactmat.2020.10.021
33. Faramarzi N, Yazdi IK, Nabavinia M, Gemma A, Fanelli A, Caizzone A, Ptaszek LM, Sinha I, Khademhosseini A, Ruskin JN, Tamayol A. Patient‐specific bio-inks for 3D bioprinting of tissue engineering scaffolds. Adv Healthc Mater. 2018;7(11):1701347. https://doi.org/10.1002/adhm.201701347
34. Pang C, Ibrahim A, Bulstrode NW, Ferretti P. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int Wound J. 2017;14(3):450-459. https://doi.org/10.1111/iwj.12735
35. Savoji H, Godau B, Hassani MS, Akbari M. Skin tissue substitutes and biomaterial risk assessment and testing. Front Bioeng Biotechnol. 2018;6:86. https://doi.org/10.3389/fbioe.2018.00086
36. Goodarzi P, Falahzadeh K, Nematizadeh M, Farazandeh P, Payab M, Larijani B, Tayanloo Beik A, Arjmand B. Tissue-engineered skin substitutes. In: Cell Biology and Translational Medicine, Volume 3: Stem Cells, Biomaterials, and Tissue Engineering, pp.143-188. DOI: 10.1007/5584_2018_226
37. Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR. Bioinks for 3D bioprinting: an overview. Biomater Sci. 2018;6(5):915-946. https://doi.org/10.1039/C7BM00765E
38. Li X, Liu B, Pei B, Chen J, Zhou D, Peng J, Zhang X, Jia W, Xu T. Inkjet bioprinting of biomaterials. Chem Rev. 2020;120(19):10793-10833. https://doi.org/10.1021/acs.chemrev.0c00008
FUNDING
No financing.
CONFLICTS OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Conceptualization: Geetika Madan Patel, Nayana Borah, Gaurav Kumar.
Methodology: Geetika Madan Patel, Nayana Borah, Gaurav Kumar.
Drafting - original draft: Geetika Madan Patel, Nayana Borah, Gaurav Kumar.
Writing - proofreading and editing: Geetika Madan Patel, Nayana Borah, Gaurav Kumar.