ORIGINAL
Aortic peak flow variation as a predictor of fluid responsiveness in pediatric septic shock patients under mechanical ventilation
Variación del flujo aórtico máximo como predictor de la respuesta a fluidos en pacientes pediátricos con shock séptico bajo ventilación mecánica
Alfredo Carlos Rodríguez-Portelles1
![]() *, Arianna Maité Céspedes Rómulo2
*, Arianna Maité Céspedes Rómulo2 ![]() *, Reynaldo Carvajal Choque3
*, Reynaldo Carvajal Choque3 ![]() *, María Paula Trujillo Pérez2
*, María Paula Trujillo Pérez2 ![]() *, Daniela Stephanie
Montenegro Salas4
*, Daniela Stephanie
Montenegro Salas4 ![]() *, Isaura Jaimes5
*, Isaura Jaimes5 ![]() *, Fátima Paola Altamirano Jara6
*, Fátima Paola Altamirano Jara6 ![]() *, Verónica Alexandra Flores Santander6
*, Verónica Alexandra Flores Santander6 ![]() *, Onelis Góngora Gómez7
*, Onelis Góngora Gómez7 ![]() *
*
1Hospital Metropolitano, Quito, Ecuador. UsPed Latinoamérica (Latin American group for teaching and research in pediatric POCUS).
2Universidad de las Américas (UDLA), Quito, Ecuador.
3Hospital Petrolero, La Paz, Bolivia. UsPed Latinoamérica (Latin American group for teaching and research in pediatric POCUS).
4Universidad Internacional SEK Ecuador, Quito, Ecuador.
5Hospital General del Instituto Ecuatoriano de Seguridad Social Quito Sur, Quito, Ecuador.
6Hospital Pediátrico “Baca Ortiz”, Universidad San Francisco de Quito, Quito, Ecuador.
7Hospital Pediátrico Docente Universitario “Octavio de la Concepción de la Pedraja, Holguín, Cuba.
Cite as: Rodríguez-Portelles AC, Céspedes Rómulo AM, Carvajal Choque R, Montenegro Salas DS, Isaura J, Altamirano Jara FP, Flores Santander VA, Góngora Gómez O. Variación del flujo aórtico máximo como predictor de la respuesta a fluidos en pacientes pediátricos con shock séptico bajo ventilación mecánica. Salud, Ciencia y Tecnología. 2023;3:584. https://doi.org/10.56294/saludcyt2023584
Submitted: 01-11-2023 Revised: 04-11-2023 Accepted: 30-12-2023 Published: 31-12-2023
Editor: Dr.
William Castillo-González ![]()
ABSTRACT
Introduction: effective fluid management is crucial in pediatric critical care, particularly for patients with septic shock. Aortic Peak Flow Variation (APFV) has emerged as a potential predictor of fluid responsiveness, yet its utility in pediatric septic shock patients under mechanical ventilation remains underexplored.
Objective: to evaluate the predictive accuracy of APFV for fluid responsiveness in pediatric septic shock patients undergoing mechanical ventilation and to establish optimal APFV cutoff values for clinical application.
Methods: in this prospective observational study conducted from January to September 2023 at the PICU of Hospital Padre Carollo “Un Canto a la Vida,” 26 pediatric septic shock patients were enrolled. Hemodynamic variables were measured before and after a standard fluid bolus of 10 ml/kg. APFV was calculated using transthoracic echocardiography, with fluid responsiveness defined as a ≥10 % increase in stroke volume index post-fluid administration. Sensitivity, specificity, and ROC curve analyses were employed to evaluate APFV’s predictive capability.
Results: out of 26 patients, 17 (65,4 %) responded to fluid administration. The mean APFV across all patients was 12,5 %. ROC curve analysis determined an APFV cutoff of 13,4 % for predicting fluid responsiveness, yielding a sensitivity of 82 % and specificity of 83 %, with an AUROC of 0,83.
Conclusions: APFV demonstrated a moderate to high level of accuracy in predicting fluid responsiveness in pediatric septic shock patients under mechanical ventilation. The identified APFV cutoff provides a practical reference for clinicians in fluid management decisions within this patient population.
Keywords: Pediatric Septic Shock; Fluid Responsiveness; Aortic Peak Flow Variation; Mechanical Ventilation, Echocardiography.
RESUMEN
Introducción: el manejo eficaz de líquidos es crucial en cuidados críticos pediátricos, particularmente para pacientes con shock séptico. La variación del flujo máximo aórtico (APFV) se ha convertido en un predictor potencial de la capacidad de respuesta a los líquidos, pero su utilidad en pacientes pediátricos con shock séptico sometidos a ventilación mecánica sigue sin explorarse.
Objetivo: evaluar la precisión predictiva de APFV para la respuesta a líquidos en pacientes pediátricos con shock séptico sometidos a ventilación mecánica .
Métodos: en este estudio observacional prospectivo realizado de enero a septiembre de 2023 en la UCIP del Hospital Padre Carollo “Un Canto a la Vida”, se inscribieron 26 pacientes pediátricos con shock séptico. Las variables hemodinámicas se midieron antes y después de un bolo de líquido estándar de 10 ml/kg de cristaloides. El APFV se calculó mediante ecocardiografía transtorácica, con la capacidad de respuesta a los líquidos definida como un aumento ≥10 % en el índice de volumen sistólico después de la administración de líquidos. Se emplearon análisis de sensibilidad, especificidad y curva ROC para evaluar la capacidad predictiva de APFV.
Resultados: de 26 pacientes, 17 (65,4 %) respondieron a la administración de líquidos. El APFV medio en todos los pacientes fue del 12,5 %. El análisis de la curva ROC determinó un punto de corte de APFV del 13,4 % para predecir la respuesta a los líquidos, lo que arrojó una sensibilidad del 82 % y una especificidad del 83 %, con un AUROC de 0,83 (0,804-0,917).
Conclusiones: APFV demostró un nivel moderado a alto de precisión en la predicción de la respuesta a los líquidos en pacientes pediátricos con shock séptico bajo ventilación mecánica. El límite de APFV identificado proporciona una referencia práctica para los médicos en las decisiones de manejo de líquidos dentro de esta población de pacientes.
Palabras clave: Shock Séptico Pediátrico; Capacidad de Respuesta a los Fluidos; Variación del Flujo Máximo Aórtico; Ventilación Mecánica, Ecocardiografía.
INTRODUCTION
Septic shock, a severe and potentially fatal manifestation of infection, presents a critical challenge in pediatric care. The pathophysiological progression of septic shock can lead to circulatory collapse, necessitating prompt and precise interventions to restore hemodynamic stability.(1) Among these interventions, fluid resuscitation remains a cornerstone in the management of pediatric septic shock. However, the administration of fluids necessitates a delicate balance, as both hypo- and hypervolemia can exacerbate the patient's condition, potentially leading to a detrimental outcome.(2,3,4)
Mechanical ventilation is often employed in the management of pediatric septic shock to maintain adequate oxygenation and ventilation.(5) However, mechanical ventilation can alter cardiac preload and afterload, thus affecting the hemodynamic response to fluid administration.(6) Consequently, identifying reliable indicators of fluid responsiveness is paramount to guide resuscitation efforts and optimize patient outcomes.
Aortic Peak Flow Variation (APFV) derived from echocardiography has emerged as a potential predictor of fluid responsiveness in mechanically ventilated patients with septic shock.(7,8) Children present a distinct physiological and hemodynamic profile compared to adults,(9) which necessitates a thorough investigation of the applicability and accuracy of APFV as a predictor of fluid responsiveness in this demographic.
The pediatric myocardium's distinct responsiveness to loading conditions, combined with the variability in vascular tone, necessitates the exploration of age-specific indices for fluid responsiveness.(10) Tailoring fluid resuscitation strategies based on reliable predictors could potentially mitigate the risk of fluid overload, which is associated with adverse outcomes including cardiac failure, pulmonary edema, and increased mortality in pediatric septic shock patients.(11,12,13)
Furthermore, the utilization of mechanical ventilation in managing pediatric septic shock introduces additional layers of complexity in assessing fluid responsiveness. The induced changes in intrathoracic pressure and its subsequent effects on cardiac filling and stroke volume are pivotal factors that could influence the accuracy of conventional static indices of preload.(14) Dynamic parameters like APFV, which encapsulates the interactions between mechanical ventilation and cardiovascular function, may offer a more precise insight into the fluid resuscitation needs of these critically ill children.(15)
This study aims to bridge this knowledge gap by evaluating the predictive value of APFV in determining fluid responsiveness among pediatric septic shock patients under mechanical ventilation. Through a prospective analysis, we intend to elucidate whether APFV can serve as a reliable, non-invasive, and real-time indicator of fluid responsiveness, thereby supporting more informed and personalized fluid resuscitation strategies in this vulnerable population. This inquiry not only aligns with the broader goal of enhancing precision medicine in critical care but also addresses an unmet need in the pediatric intensive care domain.
METHODS
Study Design and Setting: This prospective observational study was conducted in the Pediatric Intensive Care Unit (PICU) at Hospital Padre Carollo "Un Canto a la Vida”, Quito, Ecuador from January to September 2023. The study aimed to assess the predictive accuracy of APFV in estimating fluid responsiveness among mechanically ventilated pediatric septic shock patients.
Participants: A total of 26 mechanically ventilated pediatric patients with septic shock, who were admitted during the study period, were included. Eligibility criteria encompassed admitted patients from 1 to 156 months old in whom fluid administration was clinically indicated based on existing sepsis guidelines,(5) physician assessment and judgment. Mechanically ventilated without respiratory effort, with tidal volume (TV) ≥ 8 ml/kg of predicted body weight. Patients with contraindications to fluid bolus administration or with conditions affecting the reliability of APFV measurements were excluded from the study (arrhythmias, congenital cardiopathy (left-to-right shunt, patent ductus arteriosus), significant valvular heart disease, fluid overload and lack of a transthoracic window which would preclude accurate echocardiographic assessments.
Confirmation of Fluid Resuscitation Requirement: The necessity for fluid administration was confirmed based on clinical and hemodynamic signs of inadequate perfusion or hypovolemia. These signs included tachycardia, hypotension, oliguria, delayed capillary refilling, and hemodynamic instability.
Intervention: All eligible patients received a standardized fluid bolus of 10 ml/kg of saline solution 0,9 % or Ringer's lactate administered over 30 minutes. Hemodynamic variables were recorded before and after the fluid bolus administration.
Data Collection: Trans-thoracic measurements were acquired before and after the fluid bolus using a standardized protocol. The primary outcome measure was an increase in echocardiography derived stroke volume of ≥ 10 % post-fluid administration, indicating fluid responsiveness. APFV was measured alongside other hemodynamic variables before and after the fluid bolus. Data including demographic, clinical, echocardiographic, and hemodynamic variables were meticulously collected and entered a secure database for subsequent analysis.
Statistical Analysis: Data were analyzed using R Core Team (2023) v.4.3.1, Vienna, Austria and IBM SPSS Statistics for Windows, Version 28.0 v. 2021, Armonk, NY: IBM Corp. The predictive accuracy of APFV in determining fluid responsiveness was assessed using Receiver Operating Characteristic (ROC) curves. Sensitivity, specificity, and area under the ROC curve (AUC) were computed to evaluate the performance of APFV as a predictor of fluid responsiveness. Other statistical analyses included descriptive statistics and comparative analyses as appropriate. Decibans were used to determined weight of tests evidence.
Ethical Considerations: This study received ethical approval from the Institutional Review Board at Hospital Padre Carollo "Un Canto a la Vida." All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.(16)
Echocardiographic Assessment: Trans-thoracic echocardiography was performed by one of the authors with sonographic experience in critical care children, adhering to a standardized protocol, before and after the fluid bolus to measure stroke volume and other hemodynamic parameters.
APFV measurement was computed using the formula:
ΔVpeak = Vpeakmax − Vpeakmin/ (Vpeakmax+ Vpeakmin) /2 × 100
Where Vpeak max and Vpeak min are the maximum and minimum aortic peak flow velocities during one mechanical ventilation cycle, respectively. A high-resolution Samsung SonoAce R7 ultrasound machine equipped was utilized for all examinations.
Stroke volume was estimated by measuring the aortic valve area and the velocity-time integral (VTI) of blood flow across the aortic valve during systole. To enhance the precision of the APFV calculation, three consecutive measurements were taken both before and after the fluid bolus administration. The average (mean) of these three measurements was computed to obtain a single APFV value for each time point (pre- and post-fluid bolus). This approach aimed to mitigate the potential variations in APFV that may occur due to transient factors such as changes in intrathoracic pressure or heart rate. The pre- and post-fluid bolus APFV values were then utilized to evaluate the predictive accuracy of APFV in determining fluid responsiveness, as defined by a ≥ 10 % increase in stroke volume post-fluid bolus administration. For APFV measurements, the transducer was positioned on the chest of the supine patient to obtain a clear view of the aortic valve from an apical five-chamber view. The sample volume of the pulse Doppler was placed at the level of the aortic valve in the outflow tract of the left ventricle. The highest point of the tracing represents the peak flow velocity.
RESULTS
Study Population
Our prospective observational study, conducted from January to September 2023, included a cohort of 26 pediatric patients diagnosed with septic shock and undergoing mechanical ventilation in the Pediatric Intensive Care Unit of Hospital Padre Carollo "Un Canto a la Vida." The study population's mean age was 41,88 months, with a standard deviation of 33,11 months. The weight and size of the patients varied, with mean values of 13,28 kg and 91,60 cm, respectively.
Hemodynamic Responses to Fluid Administration
A key focus of our study was to assess the changes in hemodynamic variables following a standardized fluid bolus. Our primary outcome was the increase in stroke volume, with a notable responder rate of 65,4 % (17 out of 26 patients). These responders demonstrated a significant increase in stroke volume of ≥ 10 % post-fluid administration.
APFV Analysis
The mean APFV across all patients was 12,5 %. Utilizing ROC curve analysis, we established a cutoff point for APFV at 13,4 % to predict fluid responsiveness. This threshold demonstrated an AUROC 0,83 with a sensitivity of 82 % and a specificity of 83 %, indicating APFV's utility as a reliable predictor of fluid responsiveness in our pediatric cohort.
Notably, the positive and negative likelihood ratios for APFV, converted into decibans, were 6,13 dB and -6,48 dB, respectively. These figures underscored APFV's potential in clinical decision-making.
|
Table 1. Demographic and Clinical Characteristics of Study Participants |
||||
|
Variable |
Responders (n = 17) |
Non-Responders (n= 9) |
Total (n= 26) |
P-value |
|
Age (months) |
36,37(31,08) [11,50] |
56,86(36,21) [39,50] |
41,88(33,11) |
0,166 |
|
Sex (M/F) |
5(29,41 %)/12(70,59 %) |
5(71,43 %)/2(28,57 %) |
11(42,31 %)/15(57,69 %) |
_ |
|
Weight (kg) |
11,81(3,99) [5,45] |
17,30(10,29) [3,95] |
13,28(6,56) |
0,056 |
|
Size (cm) |
87,50(17,61) [12,75] |
102,71(18,25) [14,00] |
91,60(18,72) |
0,065 |
|
Body Surface Area Mosteller´s (m²) |
0,53(0,15) [0,16] |
0,69(0,25) [0,13] |
0,57(0,19) |
0,056 |
|
Septic Shock Origin |
||||
|
Respiratory (Pneumonia) |
10 (58,8 %) |
3 (33,3 %) |
13 (50 %) |
_ |
|
Neurological (Meningitis) |
2 (11,7 %) |
2 (22,2 %) |
4 (15,38 %) |
_ |
|
Sinusitis |
1 (5,9 %) |
0 (0 %) |
1 (3,85 %) |
_ |
|
Thoracic Post-Op |
4 (23,5 %) |
4 (44,4 %) |
8 (30,77 %) |
_ |
|
Ventilatory Settings |
||||
|
Tidal Volume (ml/kg) |
10,3 (0,5) |
10,4 (0,8) |
10,3 (0,6) |
0,103 |
|
PEEP (cmH2O) |
5(1) |
6 (2) |
5 (1) |
0,075 |
|
Respiratory Rate (breaths per minute) |
25 (4) |
27 (5) |
26(4) |
0,158 |
|
Inspiratory /Plateau Pressure (cmH2O) |
20(3) |
23 (6) |
21 (4) |
0,092 |
|
Vasoactive Support |
||||
|
Norepinephrine (mcg/kg/min) |
0,05 (0,02) |
0,08 (0,03) |
0,06 (0,03) |
0,062 |
|
Epinephrine (mcg/kg/min) |
0,02 (0,01) |
0,02 (0,02) |
0,02 (0,02) |
0,130 |
|
Source: Data Analysis. The numbers outside of parentheses are the mean values, numbers in parentheses are the standard deviations (SD), and numbers in square brackets are the interquartile ranges (IQR). The p-values are calculated using the two-sample t-test, comparing responders and non-responders. A p-value < 0,05 is considered statistically significant. For the categorical variable (sex), the values are presented as counts. |
||||
|
Table 2. Hemodynamic Variables Pre and Post Fluid Bolus |
|||||
|
Variable |
Pre-Bolus |
Post-Bolus |
Responders |
Non-Responders |
P-value |
|
MAP (mmHg) |
60(10)[15] |
65(12)[18] |
68(12)[15] |
59(10)[14] |
0,035 |
|
HR (beats /min) |
120(20)[25] |
115(18)[22] |
113(19)[24] |
120(15)[20] |
0,095 |
|
Stroke Volume (ml) |
25(5)[8] |
30(6)[10] |
35(6)[9] |
27(5)[7] |
0,052 |
|
Cardiac Output (L/min) |
3,0(0,5) [0,8] |
3,5(0,6) [0,9] |
3,6(0,6) [0,8] |
3,2(0,5) [0,7] |
0,078 |
|
Cardiac Index (L/min/m2) |
5,6 (0,6) [1,0] |
6,6 (0,7) [1,1] |
7,14 (0,7) [1,0] |
6,1(0,6) [0,9] |
0,061 |
|
SAP (mmHg) |
80(15)[20] |
85(16)[22] |
88(16)[20] |
82(15)[19] |
0,048 |
|
DAP(mmHg) |
40(8)[10] |
45(9)[12] |
47(9)[11] |
44(8)[10] |
0,036 |
|
SVV (%) |
14(3)[5] |
16(3)[4] |
15(2)[3] |
14(3)[4] |
0,031 |
|
ΔVao peak (%) |
12(3)[5] |
13(3)[4] |
15(3)[3] |
12(2)[4] |
0,059 |
|
VTI (cm) |
14(4)[6] |
19(5)[7] |
20(5)[6] |
16(4)[5] |
0,067 |
|
Source: statistical analysis. Each cell contains the mean value, with the standard deviation (SD) in parentheses and the interquartile range (IQR) in square brackets. P-values are illustratively calculated using the two-sample t-test, comparing the changes (post-pre) between responders and non-responders. A p-value < 0,05 is considered statistically significant. MAP (mean arterial pressure), HR (heart rate), SAP (systolic blood pressure), DAP (diastolic blood pressure), SVV (stroke volume variation), VTI (velocity time integral). |
|||||
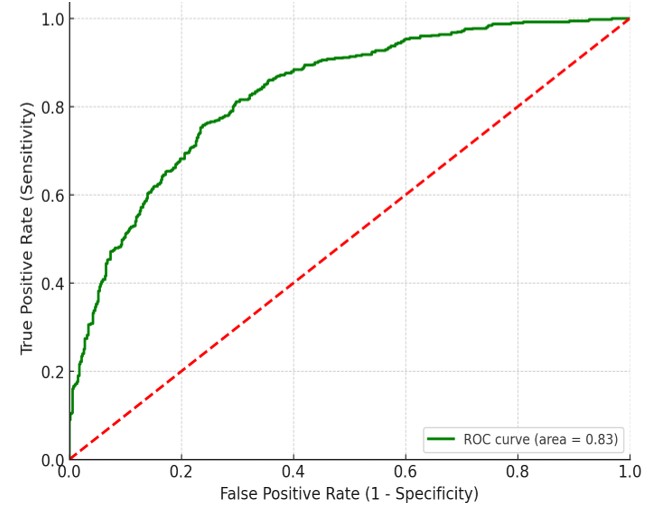
Figure 1. APFV Diagnostic ROC Curve for Pediatric Septic Shock Fluid Responsiveness
Source: statistical analysis.
ROC curve to determine fluid responsiveness with an AUC of 0,83, CI (0,804-0,917), a sensitivity of 82 %, and a specificity of 83 %. Overall, the ROC curve indicates that APFV is a valuable tool for assessing fluid responsiveness in pediatric septic shock patients.
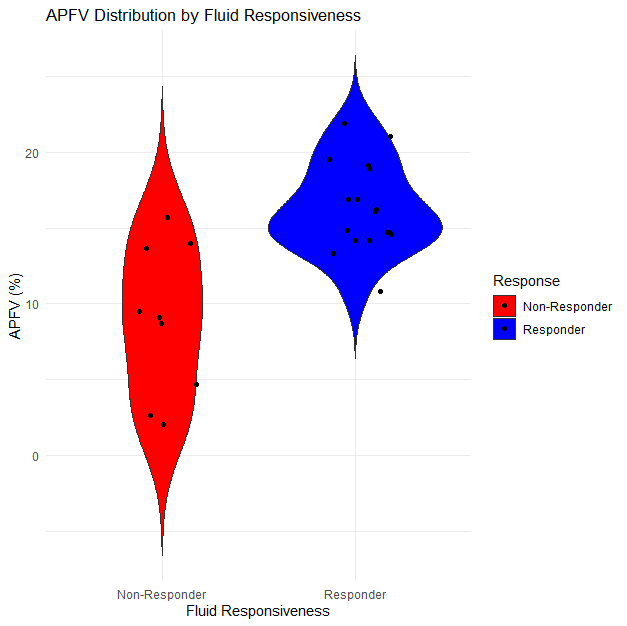
Figure 2. Violin Plot for APFV Distribution in Pediatric Septic Shock: Responders vs non-Responders
Source: statistical analysis.
The plot visualizes APFV distributions for two distinct groups – those who responded to fluid therapy (responders) and those who did not (non-responders). This differentiation is crucial in understanding how APFV values correlate with fluid responsiveness. The 'violin' shape for each group shows the density of data points at different APFV levels. A wider section of the violin indicates a higher concentration of patients at that APFV value. Conversely, a narrower section means fewer patients had APFV values in that range.
For example, if the responders’ violin is wider at higher APFV values, it suggests that responders tend to have higher APFV values. The plot also shows the median and quartile ranges for APFV within each group, offering insights into the central tendency and variability.
|
Table 3. Diagnostic Strength of APFV in Predicting Fluid Responsiveness: Odds Ratio, Likelihood Ratios, and Decibans Analysis |
||
|
Metric |
Value |
Decibans Conversion |
|
Odds Ratio (OR) |
18,22 |
- |
|
Positive Likelihood Ratio (LR+) |
4,10 |
6,13 dB |
|
Negative Likelihood Ratio (LR-) |
0,225 |
-6,48 dB |
|
Source: statistical analysis. |
||
The odds of being a responder are approximately 18 times higher for patients above the APFV cutoff than for those below it. A value of LR + 4,10 indicates that patients above the APFV cutoff are 4,1 times more likely to be true responders. The corresponding decibans value of 6,13 dB suggests a moderate increase in probability. A value of LR- 0,225 suggests that patients below the APFV cutoff are less likely to be responders. The -6,48 dB indicates a decrease in probability in decibans.
These results, imply that a higher APFV is a good predictor of fluid responsiveness. The likelihood ratios and their deciban conversions support this conclusion, with a positive LR+ indicating a good ability to identify true positives and a negative LR- suggesting a good ability to identify true negatives.
In summary, the decibans provide a logarithmic scale for interpreting the diagnostic strength of the APFV in predicting fluid responsiveness. The positive deciban value for LR+ suggests that a higher APFV is a strong predictor of fluid responsiveness, while the negative deciban value for LR- indicates that a lower APFV is indicative of non-responsiveness. These interpretations are essential for understanding the utility of APFV in clinical decision-making regarding fluid management in patients.
DISCUSSION
Our study, focused on evaluating APFV for predicting fluid responsiveness in pediatric septic shock patients, bears both notable strengths and limitations. A key strength lies in its specific focus on APFV within a well-defined pediatric population under mechanical ventilation, contributing targeted insights to the field of pediatric critical care. The use of prospective data collection and standardized fluid bolus administration enhances the reliability of our findings. However, the study's limitations include its small sample size and single-center design, which may impact the generalizability of the results. Additionally, the observational nature of the study limits our ability to infer causality. The specificity of the patient cohort, while a strength in terms of focus, may also limit the applicability of our findings to broader pediatric populations. Despite these limitations, our study adds valuable data to the growing body of research on ultrasonography as a tool for hemodynamic assessment in critically ill pediatric patients and underscores the need for further, more extensive research in this area.
A systematic review and meta-analysis by Carioca et al.(8) highlight the effectiveness of point-of-care ultrasonography in predicting fluid responsiveness in pediatric patients, primarily those under mechanical ventilation. The study's comprehensive analysis, encompassing 1028 fluid boluses across 23 studies, emphasizes the utility of respiratory variation in aortic blood flow peak velocity (ΔVpeak) as the most reliable predictor, with a notable sensitivity of 84 %, specificity of 82 %, and an AUROC of 0,89. This contrasts with our study, which focused on a smaller cohort (26 pediatric patients) and specifically assessed APFV as a predictor under mechanical ventilation. While our study achieved a sensitivity of 82 % and specificity of 83 %, our ROC was 0,83, slightly lower than the area under the summary receiver operating characteristic (AUSROC) reported in the meta-analysis. Both studies underscore the significance of ultrasonographic predictors in fluid management. However, the Carioca study(8), with its larger dataset and broader scope (including multiple predictors like ΔIVC), provides a more comprehensive understanding of POCUS's role in pediatric hemodynamic monitoring.
Another Meta-analysis from Sethasathien et al.(7) incorporated a broader dataset of 452 cases across 15 studies, found a median delta Vpeak cutoff of 12,3 % with a pooled sensitivity of 80 % and specificity of 82 %, closely paralleling our study's findings.
A major strength of the meta-analysis lies in its extensive data pool and comprehensive synthesis of diagnostic test data, offering robust evidence for the effectiveness of delta Vpeak as a predictor. Notably, the diagnostic odds ratio (DOR) of 23,41 and AUC of 0,87 underscore delta Vpeak's predictive accuracy. Our study, while more narrowly focused and with a smaller sample size, contributes to this body of evidence by providing data specific to a pediatric septic shock cohort under mechanical ventilation.
The meta-analysis by Wang et al.(17), incorporated data from 302 pediatric patients across 11 studies, reported a high pooled sensitivity (89 %) and specificity (85 %) for △VPeak, with an impressive DOR of 48 and an AUROC of 0,91. These results indicate a strong diagnostic capability of △VPeak in fluid management, which aligns with our study's findings that also highlight APFV as a reliable indicator for fluid responsiveness.
Karlsonn et al.(18), focused on respiratory variations in descending aortic flow (ΔVpeak dAo) to predict fluid responsiveness in anesthetized mechanically ventilated children. Both studies center on pediatric patients under mechanical ventilation and utilize echocardiographic parameters to predict fluid responsiveness. With its cohort of 27 patients, provides insights into the utility of ΔVpeak dAo and ΔVpeak LVOT (left ventricular outflow tract) in this context.
A key finding from this study was the moderate diagnostic power of ΔVpeak dAo, with an AUROC of 0,73 and a ΔVpeak dAo cutoff of >14 % yielding a sensitivity of 58 % and specificity of 73 %. This contrasts with our study, where the focus on APFV demonstrated a higher predictive accuracy.
Sun et al.(19) examined respiratory variations in aortic blood flow in mechanically ventilated children with leukemia and neutropenic septic shock. Focusing on a highly specific patient population, the study delves into the challenges of managing fluid therapy in children with leukemia, who often have complex clinical presentations due to their underlying condition and treatments like anthracyclines.
The study's findings,(19) showing an AUROC of 0,74 for respiratory variation in velocity time integral of aortic blood flow and 0,71 for respiratory variation in peak velocity of aortic blood flow, indicate fair reliability in predicting volume responsiveness. This is somewhat aligned with our study, which also used echocardiographic measures, specifically APFV, to predict fluid responsiveness, albeit in a broader pediatric septic shock cohort. Our study demonstrated a slightly higher predictive accuracy, possibly due to differences in patient populations and the specific echocardiographic parameters assessed.
The Banothu et al.(20) study evaluated the sensitivity and specificity of the inferior vena cava (IVC) distensibility index (∆IVC) and respiratory variation in peak aortic blood flow velocity (∆Vpeak) to predict fluid responsiveness in ventilated children with shock provided insightful parallels and contrasts to our research.
The study's findings that both ∆IVC and ΔVpeak are good predictors of fluid responsiveness, with ROC curve areas of 0,73 and 0,78 respectively, resonate with our study's conclusions on the utility of APFV in a similar context. The identified best cut-off values for ∆IVC (23 %) and ΔVpeak (11,3 %) with corresponding sensitivities and specificities contribute to the broader understanding of fluid responsiveness indicators in pediatric critical care.
Our study, while also centered on echocardiographic techniques, specifically emphasized APFV in pediatric septic shock patients, achieving a slightly higher predictive accuracy. This variance could be attributed to differences in the patient population.
The results from both studies underscore the importance of using tailored echocardiographic measures in different clinical scenarios, highlighting the need for clinicians to consider various echocardiographic parameters based on the specific clinical presentation and underlying condition of pediatric patients in shock.
CONCLUSIONS
The establishment of an APFV cutoff point for predicting fluid responsiveness has shown promise, particularly in its application to clinical decision-making. With a notable sensitivity and specificity, APFV emerges as a potentially reliable indicator for guiding fluid resuscitation strategies in critically ill pediatric patients. While our results are encouraging, they also underscore the need for further research, especially given the limitations of our study's sample size and its observational nature. Broadening the scope of research to include larger, multi-center studies could provide more definitive evidence and potentially validate APFV as a critical tool in the management of pediatric septic shock. The integration of APFV measurements into clinical practice could enhance the precision of fluid therapy, ultimately improving outcomes in this vulnerable patient population.
REFERENCES
1. Arina P, Singer M. Pathophysiology of sepsis. Current Opinion in Anesthesiology [Internet]. 2021 Apr [cited 2023 Oct 8];34(2):77. Available from: https://journals.lww.com/co-anesthesiology/abstract/2021/04000/pathophysiology_of_sepsis.4.aspx
2. Sanyaolu A, Patidar R, Ayodele O, Marinkovic A, Desai P. Pediatric Sepsis: The Importance of Understanding Criteria for Diagnosis. Pediatric Annals [Internet]. 2022 Oct [cited 2023 Oct 10];51(10):e405–8. Available from: https://journals.healio.com/doi/10.3928/19382359-20220803-07
3. San Geroteo J, Levy M, Gotchac J, Brissaud O, Dauger S. Fluid bolus therapy in pediatric sepsis: a narrative review. European Journal of Medical Research [Internet]. 2022 Nov 12 [cited 2023 Oct 12];27(1):246. Available from: https://doi.org/10.1186/s40001-022-00885-8
4. Seitz KP, Qian ET, Semler MW. Intravenous fluid therapy in sepsis. Nutrition in Clinical Practice [Internet]. 2022 [cited 2023 Oct 9];37(5):990–1003. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ncp.10892
5. Fernández-Sarmiento J, De Souza DC, Martinez A, Nieto V, López-Herce J, Soares Lanziotti V, et al. Latin American Consensus on the Management of Sepsis in Children: Sociedad Latinoamericana de Cuidados Intensivos Pediátricos [Latin American Pediatric Intensive Care Society] (SLACIP) Task Force: Executive Summary. J Intensive Care Med [Internet]. 2022 Jun 1 [cited 2023 Oct 17];37(6):753–63. Available from: https://doi.org/10.1177/08850666211054444
6. Gonzalez-Argote J. Analyzing the Trends and Impact of Health Policy Research: A Bibliometric Study. Health Leadership and Quality of Life 2023;2:28-28. https://doi.org/10.56294/hl202328
7. Sethasathien S, Jariyasakoolroj T, Silvilairat S, Srisurapanont M. Aortic Peak Flow Velocity As a Predictor of Fluid Responsiveness in Mechanically Ventilated Children: A Systematic Review and Meta-Analysis. Pediatric Critical Care Medicine [Internet]. 2023 Jul [cited 2023 Oct 18];24(7):e352. Available from: https://journals.lww.com/pccmjournal/abstract/2023/07000/aortic_peak_flow_velocity_as_a_predictor_of_fluid.19.aspx
8. Carioca F de L, de Souza FM, de Souza TB, Rubio AJ, Brandão MB, Nogueira RJN, et al. Point-of-care ultrasonography to predict fluid responsiveness in children: A systematic review and meta-analysis. Pediatric Anesthesia [Internet]. 2023 [cited 2023 Oct 21];33(1):24–37. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/pan.14574
9. Lee EP, Wu HP, Chan OW, Lin JJ, Hsia SH. Hemodynamic monitoring and management of pediatric septic shock. Biomedical Journal [Internet]. 2022 Feb 1 [cited 2023 Oct 9];45(1):63–73. Available from: https://www.sciencedirect.com/science/article/pii/S2319417021001384
10. Gonzalez-Argote J. Patterns in Leadership and Management Research: A Bibliometric Review. Health Leadership and Quality of Life 2022;1:10-10. https://doi.org/10.56294/hl202210
11. Lee JH, Kim EH, Jang YE, Kim HS, Kim JT. Fluid responsiveness in the pediatric population. Korean J Anesthesiol [Internet]. 2019 Oct [cited 2021 Nov 18];72(5):429–40. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6781210/
12.Fernández-Sarmiento J, Sierra-Zuñiga MF, González MPS, Lucena N, Lanziotti VS, Agudelo S. Association between fluid overload and mortality in children with sepsis: a systematic review and meta-analysis. BMJ Paediatrics Open [Internet]. 2023 Nov 1 [cited 2023 Nov 22];7(1):e002094. Available from: https://bmjpaedsopen.bmj.com/content/7/1/e002094
13. Márquez-González H, Casanova-Bracamontes L, Muñoz-Ramírez CM, Peregrino-Bejarano L, Bolaños-Téllez B, Yáñez-Gutiérrez L. Relation between fluid overload and mortality in children with septic shock. Arch Argent Pediatr [Internet]. 2019 Apr 1 [cited 2023 Oct 13];117(2):105–13. Available from: https://pubmed.ncbi.nlm.nih.gov/30869483/
14. Maccagnan Pinheiro Besen BA, Tomazini BM, Pontes Azevedo LC. Mechanical ventilation in septic shock. Current Opinion in Anesthesiology [Internet]. 2021 Apr [cited 2023 Oct 10];34(2):107. Available from: https://journals.lww.com/co-anesthesiology/abstract/2021/04000/mechanical_ventilation_in_septic_shock.8.aspx
15. Yenjabog P, Kanchongkittiphon W, Chutipongtanate S, Lertbunrian R, Ungprasert P. Dynamic parameters for fluid responsiveness in mechanically ventilated children: A systematic review. Frontiers in Pediatrics [Internet]. 2022 [cited 2023 Oct 9];10. Available from: https://www.frontiersin.org/articles/10.3389/fped.2022.1010600
16. Auza-Santiváñez JC, Díaz JAC, Cruz OAV, Robles-Nina SM, Escalante CS, Huanca BA. mHealth in health systems: barriers to implementation. Health Leadership and Quality of Life 2022;1:7-7. https://doi.org/10.56294/hl20227
17. Wang X, Jiang L, Liu S, Ge Y, Gao J. Value of respiratory variation of aortic peak velocity in predicting children receiving mechanical ventilation: a systematic review and meta-analysis. Crit Care [Internet]. 2019 Nov 22 [cited 2022 May 25];23(1):372. Available from: https://pubmed.ncbi.nlm.nih.gov/31757222/
18. Karlsson J, Peters E, Denault A, Beaubien-Souligny W, Karsli C, Roter E. Assessment of fluid responsiveness in children using respiratory variations in descending aortic flow. Acta Anaesthesiologica Scandinavica [Internet]. 2023 [cited 2023 Oct 10];67(8):1045–53. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/aas.14265
19. Sun S, Ren H, Wang Y, Zhang J, Li B, Ning B, et al. Respiratory Variations in Aortic Blood Flow to Predict Volume Responsiveness in Ventilated Children With Leukemia and Neutropenic Septic Shock*. Pediatric Critical Care Medicine [Internet]. 2020 May [cited 2023 Oct 18];21(5):e247. Available from: https://journals.lww.com/pccmjournal/abstract/2020/05000/respiratory_variations_in_aortic_blood_flow_to.26.aspx
20. Banothu KK, Sankar J, Pathak M, Kandasamy D, Gupta P, Kabra SK, et al. Utility of Inferior Vena Cava Distensibility and Respiratory Variation in Peak Aortic Blood Flow Velocity to Predict Fluid Responsiveness in Children with Shock. Indian J Pediatr [Internet]. 2023 Nov 1 [cited 2023 Oct 10];90(11):1077–82. Available from: https://doi.org/10.1007/s12098-023-04585-x
Financing
No external financing.
Conflict of interest
The authors declare that there are no conflicts of interest.
Authorship contribution
Conceptualization: Alfredo Carlos Rodríguez-Portelles, Arianna Céspedes Rómulo.
Formal Analysis: Alfredo Carlos Rodríguez-Portelles, Arianna Céspedes Rómulo.
Investigation: Alfredo Carlos Rodríguez-Portelles, Arianna Céspedes Rómulo, Reynaldo Carvajal Choque, María Paula Trujillo Pérez, Daniela Stephanie Montenegro Salas, Isaura Jaimes, Fátima Paola Altamirano Jara, Verónica Alexandra Flores Santander, Onelis Góngora Gómez.
Methodology: Alfredo Carlos Rodríguez-Portelles, Arianna Céspedes Rómulo, Reynaldo Carvajal Choque, María Paula Trujillo Pérez, Daniela Stephanie Montenegro Salas, Isaura Jaimes, Fátima Paola Altamirano Jara, Verónica Alexandra Flores Santander, Onelis Góngora Gómez.
Validation: Alfredo Carlos Rodríguez-Portelles, Arianna Céspedes Rómulo, Reynaldo Carvajal Choque, María Paula Trujillo Pérez, Daniela Stephanie Montenegro Salas, Isaura Jaimes, Fátima Paola Altamirano Jara, Verónica Alexandra Flores Santander, Onelis Góngora Gómez.
Visualization: Alfredo Carlos Rodríguez-Portelles, Arianna Céspedes Rómulo, Reynaldo Carvajal Choque, María Paula Trujillo Pérez, Daniela Stephanie Montenegro Salas, Isaura Jaimes, Fátima Paola Altamirano Jara, Verónica Alexandra Flores Santander, Onelis Góngora Gómez.
Writing - original draft: Alfredo Carlos Rodríguez-Portelles, Arianna Céspedes Rómulo, Reynaldo Carvajal Choque, María Paula Trujillo Pérez, Daniela Stephanie Montenegro Salas, Isaura Jaimes, Fátima Paola Altamirano Jara, Verónica Alexandra Flores Santander, Onelis Góngora Gómez.
Writing - review and editing: Alfredo Carlos Rodríguez-Portelles, Arianna Céspedes Rómulo, Reynaldo Carvajal Choque, María Paula Trujillo Pérez, Daniela Stephanie Montenegro Salas, Isaura Jaimes, Fátima Paola Altamirano Jara, Verónica Alexandra Flores Santander, Onelis Góngora Gómez.