ORIGINAL
Chemical composition and nephroprotective activity of hydroalcoholic extracts of leaves and rhizomes of Smilax purhampuy R. from Ecuador
Composición química y actividad nefroprotectora de extractos hidroalcohólicos de hojas y rizomas de Smilax purhampuy R. del Ecuador
Pilar A. Soledispa Cañarte1 ![]() *, Raisa Mangas Marín2
*, Raisa Mangas Marín2 ![]() *, Glenda M. Sarmiento Tomalá1
*, Glenda M. Sarmiento Tomalá1 ![]() *, Patricia I. Manzano Santana3
*, Patricia I. Manzano Santana3 ![]() *, Iván A. Choez Guaranda3
*, Iván A. Choez Guaranda3 ![]() *, Byron E. Zavala Soledispa4
*, Byron E. Zavala Soledispa4 ![]() *
*
1Faculty of Chemical Sciences. University of Guayaquil. “Salvador Allende” University Citadel. Ave. Kennedy S/N and Av. Delta. Guayaquil-Ecuador.
2Department of Pharmacy, Institute of Pharmacy and Food, University of Havana, La Coronela, La Lisa, Havana 13600, Cuba.
3ESPOL, Center for Biotechnological Research of Ecuador (CIBE), Campus Gustavo Galindo km 30.5 via Perimetral, P.O. Box 09-01-5863, Guayaquil-Ecuador.
4District Information and Communications Technologies Unit. Ministry of Education. Guayaquil-Ecuador.
Cite as: Soledispa Cañarte PA, Mangas Marín R, Sarmiento Tomalá GM, Manzano Santana PI, Choez Guaranda IA, Zavala Soledispa BE. Chemical composition and nephroprotective activity of hydroalcoholic extracts of leaves and rhizomes of Smilax purhampuy R. from Ecuador. Salud, Ciencia y Tecnología. 2024; 4:813. https://doi.org/10.56294/saludcyt2024813
Submitted: 25-11-2023 Revised: 04-02-2024 Accepted: 23-04-2023 Published: 24-04-2023
Editor: Prof.
Dr. William Castillo-González ![]()
ABSTRACT
Introduction: species of the genus Smilax are a vital source of potentially useful compounds that may be effective as nephroprotective agents, but there is little scientific evidence to support such claims.
Objective: to analyze the chemical composition and nephroprotective activity of hydroalcoholic extracts of leaves and rhizomes of Smilax purhampuy.
Methods: hydroalcoholic extracts were elaborated by maceration and analyzed by gas chromatography-mass spectrometry (GC-MS). The gentamicin-induced nephrotoxicity model was tested in Wistar rats at a dose of 80 mg/kg i.p. and extracts were administered orally at doses of 100, 200 and 400 mg/kg. Serum creatinine and serum urea were quantified, and histopathological observations of the kidneys were performed.
Results: in the extract of leaves, 33 compounds were identified, where the majority were palmitic, linoleic and linolenic acid. In the extract of rhizomes 23 phytoconstituents were recognized, predominantly stearic acid, dihydrocorinanteina and palmitic acid. There was evidence of a significant decrease in the level of creatinine and urea in the groups protected with extracts of leaves and rhizomes with respect to the gentamicin group in a direct relationship to the dose of the extracts. Renal histopathological changes were observed in the gentamicin group, while the groups receiving the extracts decreased the severity of damage.
Conclusions: the results indicate that Smilax purhampuy has a potential role in improving gentamicin-induced kidney damage, providing the first findings on its nephroprotective activity.
Keywords: Chemistry; GC-MS; Nephroprotective Activity; Smilax Purhampuy; Plant Leaves; Rhizomes.
RESUMEN
Introducción: las especies del género Smilax son una fuente vital de compuestos potencialmente útiles que pueden ser eficaces como agentes nefroprotectores, pero existen pocas pruebas científicas que respalden tales afirmaciones.
Objetivo: analizar la composición química y la actividad nefroprotectora de extractos hidroalcohólicos de hojas y rizomas de Smilax purhampuy.
Métodos: se elaboraron extractos hidroalcohólicos por maceración y se analizaron por cromatografía de gases-espectrometría de masas (GC-MS). Se ensayó el modelo de nefrotoxicidad inducida por gentamicina en ratas Wistar a una dosis de 80 mg/kg i.p. y se administraron extractos por vía oral a dosis de 100, 200 y 400 mg/kg. Se cuantificaron la creatinina y la urea séricas y se realizaron observaciones histopatológicas de los riñones.
Resultados: en el extracto de hojas se identificaron 33 compuestos, en su mayoría ácido palmítico, linoleico y linolénico. En el extracto de rizomas se reconocieron 23 fitoconstituyentes, predominando el ácido esteárico, la dihidrocorinanteina y el ácido palmítico. Se observó una disminución significativa del nivel de creatinina y urea en los grupos protegidos con extractos de hojas y rizomas con respecto al grupo de gentamicina, en relación directa con la dosis de los extractos. Se observaron cambios histopatológicos renales en el grupo gentamicina, mientras que los grupos que recibieron los extractos disminuyeron la severidad del daño.
Conclusiones: los resultados indican que Smilax purhampuy tiene un papel potencial en la mejora del daño renal inducido por la gentamicina, proporcionando los primeros hallazgos sobre su actividad nefroprotectora.
Palabras clave: Química; CG-EM; Actividad Nefroprotectora; Hojas; Rizomas; Smilax Purhampuy.
INTRODUCTION
Medicinal plants are a promising source of new compounds potentially useful for the treatment of kidney problems, since the effectiveness of some plant species has been demonstrated as nephroprotective agents that can mitigate processes such as interstitial nephritis, alteration of intraglomerular hemodynamics, tubular necrosis or glomerulonephritis. However, scientific evidence to support such claims is scarce.(1,2,3)
Nephrotoxicity is one of the most common kidney problems and can be defined as a kidney disease or dysfunction that occurs when our body is directly or indirectly exposed to harmful drugs and industrial or environmental chemicals, because of this, the kidneys become highly susceptible. Several toxic agents are responsible for nephrotoxicity, including gentamicin, widely used for the treatment of gram-negative bacterial infections. However, its nephrotoxicity and ototoxicity are the main limitations in clinical use.(4) Therefore, it is necessary to search for natural alternatives to mitigate such damage.
The genus Smilax belongs to the family Smilacaceae and is distributed in tropical and subtropical regions. The species are widely used in traditional medicine for the treatment of rheumatism, syphilis and as a diuretic. Previous research has pointed to several biological activities of Smilax species extracts, such as anti-inflammatory, antinociceptive, antifungal, estrogenic, diuretic, and antihyperuricemic properties.(5,6) However, nephroprotective activity has been little explored.
Smilax purhampuy is native to the Amazon region, distributed throughout Ecuador, Peru, Nicaragua, Colombia, Bolivia, Costa Rica, Venezuela, Honduras and Brazil.(7) Traditionally, it is used to lower cholesterol and triglycerides, in the treatment of chronic gastritis, cystitis, arthritis and inflammation of the prostate.(8) Recent studies demonstrated the anti-inflammatory efficacy of hydroalcoholic extracts of leaves and rhizomes on the model of acute inflammation induced by carrageenan, as well as the presence of phenolic compounds and triterpenoids.(9)
Taking into consideration the limited phytochemical and biological studies and the need to find new options for the prevention of induced renal toxicity, the objective of the present study was to analyze the chemical composition and nephroprotective activity of hydroalcoholic extracts of leaves and rhizomes of Smilax purhampuy.
METHODS
When working with experimental animals, the ethical principles based on the 3Rs were considered from the beginning to the end, guaranteeing their well-being and protection and reaching the validity of the results obtained, such is the case for carrying out the euthanasia, where rats were subjected to an ether-saturated atmosphere. In addition, the established Bioethics and Biosafety Standards were complied with The World Medical Association.(10)
Plant material
S. purhampuy Ruiz was collected in the months of May and April 2019 from specimens from the Francisco de Orellana province in Ecuador. The samples were transferred to the GUAY herbarium of the Faculty of Natural Sciences of the University of Guayaquil where it was assigned the identification code 13,117. Genetic characterization of the species was also performed.(11)
From the collections, the leaves and rhizomes were used, washed with drinking water and dried in a Mettler Toledo oven (Switzerland) at 40 °C ± 2 °C, up to constant weight. The dried samples were crushed in a hand-crafted blade mill and stored in amber glass jars for further analysis.
Obtaining the extracts
The extracts of leaves and rhizomes of S. purhampuy were prepared by maceration (seven days), with sporadic shaking at a temperature of 30 ± 2 ° C at a rate of 20 g of drug / 100 mL of solvent,(12) with the use of 80 % hydroalcoholic mixture as menstruum. For the biological test the extracts were concentrated under reduced pressure in a rotoevaporator (IKA RV, China) at 40 °C and in order to facilitate the dosage of the extract they were resuspended in 0,5 % carboxymethylcellulose (Sigma Aldrich).
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The extracts of leaves and rhizomes were analyzed in a gas chromatograph Agilent Technologies (California, USA) coupled to Agilent 5975C mass spectrometer with ionization source with electronic impact and single quadruple analyzer. Prior to analysis, samples were derivatized with N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA), Sigma-Aldrich (100 μL of the derivatizing agent was added in 100 μL of dry sample and placed in water bath at 80 °C for 2 hours). The working conditions were injector temperature 250 °C, injection volume 2 μL; helium carrier gas at 1,2 mL/min; DB-5MS column (30 m length × 0,25 mm internal diameter and 0,25 micrometers film thickness); initial temperature 70 °C for 2 minutes increasing 5 °C / min to 300 °C for 6 min. Mass spectrometer operated at 70 eV in full scan mode from 50 to 550 mass units. Source temperature 230 °C, quadrupole temperature 150 °C.(13) The compounds were identified by comparing their mass spectra and the Wiley 9th mass reference with NIST 2011 MS Library taking into account those with a similarity percentage of 95 % or greater.
Nephroprotective activity
The nephroprotective activity wasverified using the gentamicin-induced acute nephrotoxicity model in rats by intraperitoneal injection at a dose of 80 mg / kg of body weight, as described by Abdel-Raheem.(14)
We used 40 adults male Wistar albino rats weighing between 160 and 210 g, from the National Center for the Production of Laboratory Animals (Mayabeque, Cuba) with their corresponding quality certificates that guaranteed their health, being suitable for this type of test.
The animals were housed in the vivarium of the Center for Studies for Biological Research and Evaluations of the Institute of Pharmacy and Food (CIEB-IFAL) of the University of Havana with a local temperature of 20 ± 3 ° C, relative humidity of 30 - 70 % ± 5 %, light / dark cycle of 12 / 12 h. Water and food were provided "ad libitum". The sanitation of the boxes and the replacement of the beds were guaranteed, the overcrowding of the animals, exposure to noise and other factors that could cause stress were avoided.
The rats were divided into eight equal groups consisting of five animals each: Group 1: sodium chloride (0,9 %), 5 mL/kg body weight (BW) orally (normal control); Group 2: gentamicin (Empresa Laboratorios AICA, Cuba), 80 mg/kg BW intraperitoneally (IP) (negative control); Groups 3, 4, 5: leaf extracts at doses of 100, 200 and 400 mg/kg BW, respectively, with gentamicin IP, 80 mg/kg BW.; Group 6,7,8: rhizome extracts at doses of 100, 200 and 400 mg/kg, respectively, with gentamicin IP, 80 mg/kg BW The animals received gentamicin for seven days to induce nephrotoxicity and were consecutively administered with different doses of the extracts in the morning hours.
At the end of the experiment (24 h after the last administration of the treatments), all rats were sacrificed in a chamber saturated with ether. Blood was drawn from the heart for the estimation of renal function tests: serum urea (using the Urea SaliUrea-test kit, Cuba) and serum creatinine (creatinine kit, Cuba). The determinations were made on a spectrophotometer (VS-820, Cuba) at 500 nm and 620 nm, respectively. The elevation of urea and creatinine levels in serum was taken as an index of nephrotoxicity.(14,15)
Both kidneys were isolated from the rats and divided into two longitudinal sections. Half of each kidney was fixed in 10 % buffered neutral formalin solution (Sigma-Aldrich) and then embedded in paraffin and finally cut into a Kedee manual microtome (model KD-202A, China). Dewaxed sections (5-8 μm) were stained with hematoxylin and eosin (Sigma-Aldrich).(16) Histological observations were performed using a NOVEL optical microscope (China) (10X and 20X lenses).
All biological experiments were carried out following the provisions of the standard operating procedures (SOP) in force at the Study Center for Biological Research and Evaluations of the Institute of Pharmacy and Food of the University of Havana. The refinement techniques that govern animal experimentation were also taken into account, guaranteeing their welfare and protection, complying with the instructions recommended in the International Guidelines.(17,18)
Statistical analysis
Pharmacological test data were analyzed by single-way ANOVA, followed by a Tukey's multiple mean comparisons test, considering significant differences p ≤ 0,05. For the processing and statistical analysis of the results, the statistical program SPSS for Windows version 8.0 was used. Experimental values were expressed as mean/standard deviation (SD).
RESULTS
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
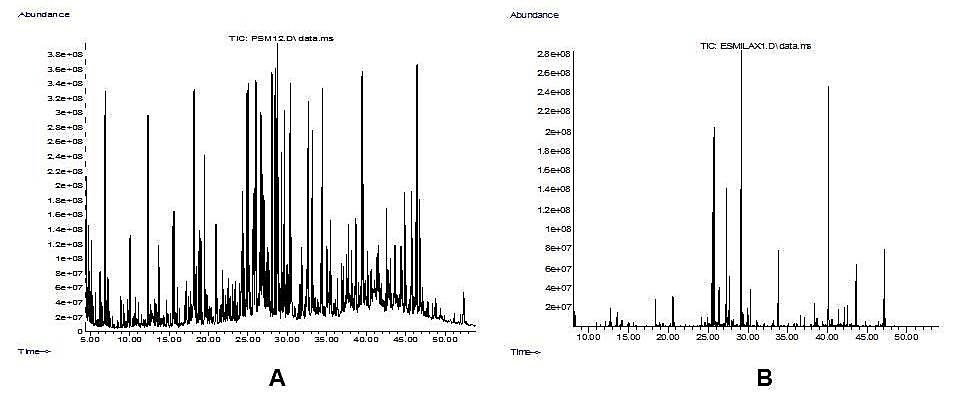
In the extract of leaves, 33 compounds were identified, where the majority were palmitic, linoleic and linolenic acid. The extract of rhizomes was characterized by the presence of 23 phytoconstituents, predominantly stearic acid, dihydrocorinanteina and palmitic acid. Figure 1 illustrates the analytical gas chromatograms of hydroalcoholic extracts of leaves and rhizomes of S. purhampuy Ruiz and table 1 shows the compounds identified.
|
|
|
Figure 1. Analytical gas chromatograms of hydroalcoholic extracts S. purhampuy Ruiz. (A) Leaf extract; (B) Rhizome extract |
|
Table 1. Compounds identified in the hydroalcoholic extract of leaves and rhizomes of S. purhampuy Ruiz |
|||||||
|
Rhizome Extract |
Leaf Extract |
||||||
|
No. |
TR (min.) |
Compounds |
AR (%) |
No |
TR (min.) |
Compounds |
ON (%) |
|
1 |
13,34 |
L-Prolina |
0,04/0,00 |
1 |
5,68 |
Propane- 1,2 diol |
0,15/0,01 |
|
2 |
14,30 |
Propanoic acid |
0,03/0,00 |
2 |
6,48 |
Butano |
0,04/0,00 |
|
3 |
15,09 |
Serina |
0,15/0,00 |
3 |
8,86 |
3-Hydroxypropionic acid |
0,10/0,01 |
|
4 |
18,36 |
Succinic acid |
1,16/0,01 |
4 |
9,73 |
Phosphoric acid |
0,18/0,02 |
|
5 |
19,19 |
α-Proline-5-oxo-methyl ester |
0,06/0,04 |
5 |
13,10 |
Thymol |
0,11/0,02 |
|
6 |
20,27 |
n-Dodecanol |
0,14/0,00 |
6 |
13,57 |
Butanedioic acid |
0,05/0,01 |
|
7 |
21,45 |
Glutamic acid |
0,03/0,00 |
7 |
13,70 |
Glyceric acid |
0,29/0,01 |
|
8 |
21,68 |
Ribonic acid-γ-lactone |
0,03/0,00 |
8 |
16,34 |
3,4-dihydroxybutanoic acid |
0,07/0,00 |
|
9 |
22,17 |
Lauric acid |
0,12/0,00 |
9 |
17,49 |
Arabino-hexos-2-ulosa |
0,21/0,07 |
|
10 |
22,48 |
D-Arabinopyranose |
0,03/0,00 |
10 |
17,72 |
Malic acid |
0,27/0,01 |
|
11 |
24,64 |
Tetradecan |
0,28/0,00 |
11 |
18,36 |
Succinic acid |
0,11/0,01 |
|
12 |
25,81 |
Citric acid |
0,32/0,00 |
12 |
19,20 |
D-Eritro-pentitol, 2-deoxi |
0,06/0,00 |
|
13 |
25,92 |
α-D-Talapiranosa |
0,26/0,00 |
13 |
21,04 |
D-(+)-Ribono-1,4-lactone |
0,56/0,00 |
|
14 |
26,38 |
L-Altrosa |
2,47/0,03 |
14 |
23,05 |
L-(-)-Arabitol |
0,20/0,01 |
|
15 |
28,00 |
Glucitol |
0,30/0,12 |
15 |
23,15 |
Ribitol |
0,13/0,01 |
|
16 |
28,41 |
D+thafuranose |
0,06/0,00 |
16 |
25,30 |
D-(-)-Fructofuranosa |
0,15/0,01 |
|
17 |
30,29 |
Palmitic acid |
1,65/0,01 |
17 |
26,45 |
D-gluonic acid |
0,37/0,00 |
|
18 |
31,01 |
Inositol |
0,31/0,02 |
18 |
27,27 |
Palmitic acid methyl ester |
0,32/0,01 |
|
19 |
31,30 |
Nonacosanol |
0,06/0,00 |
19 |
27,32 |
D-manitol |
0,37/0,01 |
|
20 |
33,30 |
Oleic acid |
0,10/0,00 |
20 |
30,29 |
Palmitic acid |
1,66/0,04 |
|
21 |
33,86 |
Stearic acid |
3,97/0,04 |
21 |
31,37 |
Margaric acid |
0,13/0,04 |
|
22 |
43,56 |
Dihydrocorinanteina |
2,82/0,01 |
22 |
31,59 |
Tributil aconitato |
0,25/0,03 |
|
23 |
48,40 |
Stigmasterol |
0,05/0,00 |
23 |
31,79 |
Decanedioic acid dibutyl ester |
0,35/0,03 |
|
|
|
|
|
24 |
32,50 |
Linoleic acid |
0,46/0,03 |
|
|
|
|
|
25 |
32,62 |
α linolenic acid |
1,40/0,06 |
|
|
|
|
|
26 |
35,01 |
9-octadecenamide |
0,46/0,09 |
|
|
|
|
|
27 |
36,34 |
Uridine |
0,32/0,06 |
|
|
|
|
|
28 |
40,27 |
Maltose |
0,27/0,03 |
|
|
|
|
|
29 |
40,67 |
2α –Mannobiosa |
0,32/0,02 |
|
|
|
|
|
30 |
41,36 |
13-Docosenamida |
0,36/0,01 |
|
|
|
|
|
31 |
41,88 |
3-α-Mannobiosa |
0,24/0,02 |
|
|
|
|
|
32 |
44,22 |
Galactinol |
0,13/0,02 |
|
|
|
|
|
33 |
49,39 |
D-(+)-Turanosa |
0,09/0,01 |
|
Note: RT= retention time, x̅/SD= Average value of determinations/standard deviation (n=3) |
|||||||
Nephroprotective activity
The nephroprotective efficacy of hydroalcoholic extracts of leaves and rhizomes of S. purhampuy Ruiz, administered for seven days, was demonstrated by reversing the damage caused by gentamicin, which was evidenced in a decrease in serum levels of urea and creatinine. Table 2 shows the results of the study. There were significant differences in the groups supplied with the extracts at different doses with respect to the group treated with gentamicin, achieving a concentration dependent nephroprotective effect, where the lower values of urea and creatinine were appreciated at the highest dose evaluated, both leaves and rhizomes.
|
Table 2. Nephroprotective activity of hydroalcoholic extracts of leaves and rhizomes of S. purhampuy Ruiz |
|||
|
Groups |
Parameters |
||
|
Urea (mg/dL) |
Creatinine (mg/dL) |
||
|
NaCl 0,9 % |
22,7 ± 1,78a |
0,57/0,04a |
|
|
(Gentamicin 80 mg/kg |
52,16 ± 1,07b |
4,20/0,24b |
|
|
Leaf extract |
100 mg/kg |
32,86 ± 2,54c |
2,06/0,008c |
|
200 mg/kg |
30,00 ± 0,77d |
1,77/0,21d |
|
|
400 mg/kg |
25,81 ± 1,64e |
1,14/0,05e |
|
|
Rhizome extract |
100 mg/kg |
36,12/2,95f |
2,47/0,20f |
|
200 mg/kg |
31,04/0,86cd |
2,05/0,10c |
|
|
400 mg/kg |
28,78/0,99d |
1,60/0,17d |
|
|
The results are expressed as the mean of the determinations ± standard deviation. (n=5). Different letters in a column indicate significant differences, according to Tukey's test (p ≤ 0,05). |
|||
Histopathological studies demonstrated that gentamicin-induced acute nephrotoxicity in rats was characterized by the presence of glomerular congestion, peritubular congestion, epithelial desquamation of the proximal tubule, congestion of blood vessels, and large numbers of inflammatory cells in the negative control group. In the normal control groups and those administered with extracts, both leaves and rhizomes at doses of 200 and 400 mg / kg, no kidney involvement was perceived. However, in the groups treated with doses of 100 mg/kg extract, some inflammatory cells were observed; However, at this dose it was also possible to reduce serum levels of urea and creatinine, demonstrating its nephroprotective effect.
DISCUSSION
The GC-MS study revealed that S. purhampuy is an important source of metabolites. The leaf extract had a composition richer in phytochemicals, characterized by the presence of saturated and unsaturated fatty acids, low molecular weight alcohols, organic and inorganic acids, sugars, an alkane, and a monoterpene. For its part, the extract of rhizomes presented amino acids, alcohols of high molecular weight, organic acids, saturated and unsaturated fatty acids, sugars, an alkaloid, and a sterol.
Among the major and common compounds for both extracts with a similar relative abundance, palmitic acid was found, which has recognized anti-inflammatory activity through the inhibition of phospholipase A2, one of the mediators of the inflammatory process.(19) The prevalence of linoleic and linolenic acid in the leaf extract shows the therapeutic potential of the species. Both fatty acids directly inhibit inflammation by competing with arachidonic acid or indirectly by affecting transcription factors or nuclear receptors responsible for inflammatory gene expression.(20) The majority presence of palmitic, linoleic and lyolenic acid is in correspondence with studies carried out on extracts of Smilax brasilensis leaves,(21) as well as the presence of stearic acid and stigmasterol (although minority) has been identified in the rhizomes of Smilax canellifolia.(22)
The notorious aspect of the study was the fact of finding the alkaloid dihydrocorinanteina that has reported significant leishmanicidal activity in vitro(23) and has not been previously reported in the genus Smilax. All the compounds identified constitute new reports for the species.
In the present research, the nephroprotective action of hydroalcoholic extracts of leaves and rhizomes of S. purhampuy Ruiz administered orally at doses of 100, 200 and 400 mg / kg of body weight for seven days was evaluated, considering antecedents of the same study with other plant extracts of the same genus.(24)
Gentamicin was used as an agent inducing nephrotoxicity; This drug is an aminoglycoside antibacterial that is commonly used worldwide for the treatment of infections caused by Gram-negative bacteria;(25,26,27) however, it has significant nephrotoxic potential in humans and experimental animals, so it is widely used as a model to study nephrotoxicity.(15,26,27)
The pathological mechanism of gentamicin-induced nephrotoxicity includes oxidative stress, apoptosis, necrosis, and increased monocytes/macrophage infiltration. Gentamicin-induced nephrotoxicity is associated with renal oxidative stress in the kidney due to overproduction of reactive oxygen species, reduction in renal antioxidant defense mechanism, and results in glomerular damage, renal inflammation, and tubular necrosis among the important complications.(2,4,28)
Supplementation with several doses of extracts of leaves and rhizomes of S. purhampuy Ruiz to rats treated with gentamicin, caused a decrease in urea and creatinine levels, being more significant at the dose of 400 mg / kg where the leaf extract showed the lowest values, which evidences the dose dependent nephroprotective effect. Rats treated with gentamicin alone produced a typical pattern of nephrotoxicity manifested by a marked increase in serum creatinine and serum urea.(29) It is known that urea and creatinine are evaluative parameters of renal function, they are wastes resulting from metabolism that are excreted by the kidneys and thus compared.(30)
The histological observations correspond to the renal parameters evaluated in the negative control group where a significant decrease in renal functions was perceived compared to the normal control groups and those that were administered with the extracts, mainly at the highest doses evaluated (200 and 400 mg / kg). Similar results were obtained by Unis and Abdelbary(27) in which they showed that the groups of rats that received green coffee bean extract orally for seven days, after a daily intraperitoneal injection of gentamicin for seven days, manifested a significant improvement in kidney function tests compared to the group treated with gentamicin.
The results of the present study are also consistent with those obtained by Özsoy (24) in evaluations carried out at various doses (100, 200 and 400 mg/kg body weight) of an aqueous extract of Smilax excelsa leaves, but in a model of carbon tetrachloride-induced nephrotoxicity, where it was observed that at the highest dose the nephrotoxic effect was reversed. Nephroprotective activity has also been reported for S. china.(31)
The nephroprotective effect of the extracts could be related to the phytochemicals detected for the species under study. Previous research by Soledispa(9) on hydroalcoholic extracts of leaves and rhizomes of S. purhampuy demonstrated the presence of phenolic compounds. Several investigations have shown that phenolic compounds (phenolic acids, flavonoids) have nephroprotective activity through their potent antioxidant effect, and in models of nephrotoxicity induced by gentamicin(32) and by carbon tetrachloride.(33)
Research with omega-3 fatty acids suggested that these are effective in reducing proteinuria in patients with chronic glomerular disease and that action is dose-dependent, thereby improving kidney function. (34) Xie demonstrated the nephroprotective effect of eight amino acids, including L-proline and serine, against cisplatin-induced nephrotoxicity.(35)
In the literature, this is the first scientific evidence exploring the effect of S. purhampuy Ruiz on gentamicin-induced acute nephrotoxicity in rats. The present study demonstrated that the administration of hydroalcoholic extracts of leaves and rhizomes protects against the deterioration of renal functions induced by gentamicin under the test conditions. Further studies are needed to elucidate the exact cellular mechanism of the demonstrated nephroprotective effect.
CONCLUSIONS
In the present study, for the first time, according to the literature consulted, the presence of fatty acids, organic and inorganic acids, alcohols and sugars in the extracts of leaves and rhizomes of Smilax purhampuy is reported. The hydroalcoholic extracts of leaves and rhizomes of S. purhampuy Ruiz at the three doses tested were effective as nephroprotective agents and may be a potential therapeutic option in the effective management of gentamicin-induced nephrotoxicity.
REFERENCES
1. Srivastava A, Kaushik D, Shrivastava A, Lal V. Nephroprotective ethno-medicinal action of selected Indian medicinal plants. International Journal of Pharmaceutical Sciences and Drug Research [Internet]. 29 de marzo de 2017;9(2). Available in: https://doi.org/10.25004/ijpsdr.2017.090202
2. Manshare K, Anand A, Mahajan S, Mehta M. Evaluation of nephroprotective activity of gallic acid in gentamicin-induced rat model of nephrotoxicity. ResearchGate [Internet]. 1 de julio de 2018; Available in: https://www.researchgate.net/publication/326673422_Evaluation_of_Nephroprotective_Activity_of_Gallic_Acid_in_Gentamicin-induced_rat_Model_of_Nephrotoxicity
3. Tienda-Vázquez MA, Morreeuw ZP, Sosa‐Hernández JE, Cardador‐Martínez A, Sabath E, Melchor-Martínez EM, et al. Nephroprotective plants: A review on the use in Pre-Renal and Post-Renal diseases. Plants [Internet]. 18 de marzo de 2022;11(6):818. Available in: https://doi.org/10.3390/plants11060818
4. Negi K, Mirza AH. Nephroprotective and therapeutic potential of traditional medicinal plants in renal diseases. Journal of drug research in ayurvedic sciences [Internet]. 1 de enero de 2020;5(3):175-83. Available in: https://doi.org/10.5005/jdras-10059-0079
5. Hirota BCK, Da Silva Paula C, De Oliveira VB, Da Cunha JM, Schreiber AK, Ocampos FMM, et al. Phytochemical and Antinociceptive, Anti-Inflammatory, and antioxidant studies of Smilax larvata (Smilacaceae). Evidence-based Complementary and Alternative Medicine [Internet]. 1 de enero de 2016;2016:1-12. Available in: https://doi.org/10.1155/2016/9894610
6. Da Cunha APS, Ferreira LS, Gluzezak AJP, Petrica EEA, Sinhorin AP, Sinhorin VDG. Investigation of the possible protective effect of Smilax fluminensis steud. leaf in mice subjected to oxidative stress by paracetamol. Bioscience Journal [Internet]. 29 de diciembre de 2021;37:e37070. Available in: https://doi.org/10.14393/bj-v37n0a2021-53862
7. Global Biodiversity Information Facility (GBIF). Smilax Purhampuy Ruiz [Internet]. Copenhagen: GBIF; 2012. Available in: https://www.gbif.org/species/5295631
8. JR Global del Perú S.A.C. Ficha Técnica-Zarzaparrilla - Inkaplus [Internet]. yumpu.com. Available in: https://www.yumpu.com/es/document/read/15083310/zarzaparrilla-inkaplus
9. Soledispa-Cañarte P, Mangas-Marín R, Gutiérrez-Gaitén Y, Sarmientos-Tomalá G. Perfil fitoquímico y actividad antiinflamatoria de hojas y rizomas de Smilax purhampuy Ruiz. Revista Cubana de Farmacia [Internet]. 2022;55(1). Available in: https://revfarmacia.sld.cu/index.php/far/article/view/742/464
10. Graham ML, Prescott MJ. The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease. European Journal Of Pharmacology [Internet]. 1 de julio de 2015;759:19-29. Disponible en: https://doi.org/10.1016/j.ejphar.2015.03.040
11. Soledispa PA, Santos-Ordóñez E, Miranda M, Pacheco R, Gaitén YIG, Scull R. Molecular barcode and morphological analysis of Smilax Purhampuy Ruiz, Ecuador. PeerJ [Internet]. 18 de marzo de 2021;9:e11028. Available in: https://doi.org/10.7717/peerj.11028
12. Miranda, M.M. and Cuéllar, A.C. (2000) Manual de prácticas de laboratorio. Farmacognosia y Productos Naturales. Ciudad Habana, 25-49, 74-79. - References - Scientific Research Publishing [Internet]. Available in: https://www.scirp.org/reference/referencespapers?referenceid=2380444
13. López LA, Chóez-Guaranda I, Lavid GAC, Martínez MM. Pharmacognostic study and evaluation of the antioxidant capacity of the fruit of two varieties of Nephelium lappaceum L. (Sapindaceae), (Rambutan). Journal of Pharmacy & Pharmacognosy Research [Internet]. 1 de enero de 2020;8(1):64-77. Available in: https://doi.org/10.56499/jppres19.701_8.1.64
14. Abdel-Raheem IT, Abdelghany AA, Mohamed GA. Protective effect of Quercetin against Gentamicin-Induced nephrotoxicity in rats. Biological & Pharmaceutical Bulletin [Internet]. 1 de enero de 2009;32(1):61-7. Available in: https://doi.org/10.1248/bpb.32.61
15. Chandavarkar S, Desai SM, Gautam GK. Nephroprotective activity of different extracts of Biophytum sensitivum (Linn.) DC. International Journal of Herbal Medicine [Internet]. 1 de enero de 2017;5(1):31-4. Available in: https://www.florajournal.com/archives/2017/vol5issue1/PartA/4-3-8-384.pdf
16. Elsebay SAG, Abdelnaby EA, Khalaf G, Abou-Rabia NM. Characterization of the decellularized male rabbit kidney as a Three-Dimensional natural scaffold for tissue engineering. a histological study. The Egyptian Journal of Histology [Internet]. 20 de agosto de 2021;0(0):0. Available in: https://doi.org/10.21608/ejh.2021.84255.1524
17. Diehl K h., Hull R, Morton DB, Pfister R, Rabémampianina Y, Smith D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. Journal of Applied Toxicology [Internet]. 1 de enero de 2001;21(1):15-23. Available in: https://doi.org/10.1002/jat.727
18. Lilley E, Stanford SC, Kendall D, Alexander S, Cirino G, Docherty JR, et al. ARRIVE 2.0 and The British Journal of Pharmacology: Updated Guidance for 2020. British Journal of Pharmacology [Internet]. 14 de julio de 2020;177(16):3611-6. Available in: https://doi.org/10.1111/bph.15178
19. Aparna VS, Dileep KV, Mandal PK, Ponnuraj K, Sadasivan C, Haridas M. Anti‐Inflammatory property of N‐Hexadecanoic acid: Structural evidence and kinetic assessment. Chemical Biology & Drug Design [Internet]. 27 de junio de 2012;80(3):434-9. Available in: https://doi.org/10.1111/j.1747-0285.2012.01418.x
20. Ahmad TB, Rudd D, Kotiw M, Лю Л, Benkendorff K. Correlation between fatty acid profile and Anti-Inflammatory activity in common Australian seafood by-Products. Marine Drugs [Internet]. 6 de marzo de 2019;17(3):155. Available in: https://doi.org/10.3390/md17030155
21. Amado PA, Ferraz V, Silva DB, Carollo CA, Castro AHF, Lima LARDS. Chemical composition, antioxidant and cytotoxic activities of extracts from the leaves of Smilax brasiliensis Sprengel (Smilacaceae). Natural Product Research [Internet]. 15 de mayo de 2017;32(5):610-5. Available in: https://doi.org/10.1080/14786419.2017.1327861
22. Peddie DA, Bryan S, Francis S, Alexander-Lindo R. Hypoglycaemic activity of Smilax canellifolia Mill. rhizomes: A bioassay-guided isolation and identification of synergistic compounds. Clinical Phytoscience [Internet]. 18 de noviembre de 2021;7(1). Available in: https://doi.org/10.1186/s40816-021-00325-w
23. Jiménez-Arellanes MA, Alamilla-Fonseca L, Gutiérrez-Rebolledo GA. Las plantas medicinales de México como fuente de compuestos activos contra la leishmaniasis. Revista mexicana de ciencias farmacéuticas [Internet]. 1 de junio de 2014;45(2):19-30. Available in: https://www.redalyc.org/pdf/579/57932294003.pdf
24. Özsoy N, Okyar A, Arda-Pirinçci P, Can A, Bolkent Ş, Akev N. Deneysel olarak oluşturulmuş böbrek hasarı üzerine Smilax Excelsa L. kullanımının değerlendirilmesi. Kafkas Universitesi Veteriner Fakultesi Dergisi [Internet]. 1 de enero de 2013; Available in: https://doi.org/10.9775/kvfd.2013.9253
25. Friesen WJ, Johnson B, Sierra J, Jin Z, Vazirani P, Xue X, et al. The minor gentamicin complex component, X2, is a potent premature stop Codon readthrough molecule with therapeutic potential. PLOS ONE [Internet]. 25 de octubre de 2018;13(10):e0206158. Available in: https://doi.org/10.1371/journal.pone.0206158
26. Becerra BK, García DJ, Becerra BM, Ruiz RE, Chávez RL. Efecto nefroprotector del Camu Camu (Myrciaria dubia) en un modelo de nefrotoxicidad inducida por gentamicina en ratas. Revista chilena de nutrición [Internet]. 1 de junio de 2019;46(3):303-7. Available in: https://doi.org/10.4067/s0717-75182019000300303
27. Unis A, Abdelbary A. Green coffee bean extract attenuates gentamicin induced acute nephrotoxicity in rats. Latin American and Caribbean Bulletin of Medicinal and Aromatic Plants [Internet]. 30 de marzo de 2022;21(2):256-68. Available in: https://doi.org/10.37360/blacpma.22.21.2.16
28. Auza-Santivañez JC, Lopez-Quispe AG, Carías A, Huanca BA, Remón AS, Condo-Gutierrez AR, et al. Work of the emergency system in polytraumatized patients transferred to the hospital. AG Multidisciplinar 2023;1:9-9. https://doi.org/10.62486/agmu20239.
29. Gonzalez-Argote J, Castillo-González W. Update on the use of gamified educational resources in the development of cognitive skills. AG Salud 2024;2:41-41. https://doi.org/10.62486/agsalud202441.
30. Marcillí MI, Fernández AP, Marsillí YI, Drullet DI, Isalgué VMF. Characterization of legal drug use in older adult caregivers who are victims of violence. SCT Proceedings in Interdisciplinary Insights and Innovations 2023;1:13-13. https://doi.org/10.56294/piii202313.
31. Machuca-Contreras F, Lepez CO, Canova-Barrios C. Influence of virtual reality and augmented reality on mental health. Gamification and Augmented Reality 2024;2:25-25. https://doi.org/10.56294/gr202425.
32. Quiroz FJR, Gamarra NH. Psychometric evidence of the mobile dependence test in the young population of Lima in the context of the pandemic. AG Salud 2024;2:40-40. https://doi.org/10.62486/agsalud202440.
33. Marcillí MI, Fernández AP, Marsillí YI, Drullet DI, Isalgué RF. Older adult victims of violence. Satisfaction with health services in primary care. SCT Proceedings in Interdisciplinary Insights and Innovations 2023;1:12-12. https://doi.org/10.56294/piii202312.
34. Auza-Santiváñez JC, Díaz JAC, Cruz OAV, Robles-Nina SM, Escalante CS, Huanca BA. Gamification in personal health management: a focus on mobile apps. Gamification and Augmented Reality 2024;2:31-31. https://doi.org/10.56294/gr202431.
35. Cuervo MED. Exclusive breastfeeding. Factors that influence its abandonment. AG Multidisciplinar 2023;1:6-6. https://doi.org/10.62486/agmu20236.
36. Chilwant, K. S., & Muglikar, A. G. Effect of honey on gentamicin induced nephrotoxicity in albino rats. International journal of pharma and bio sciences [Internet]. 1 de enero de 2012; Available in: https://www.ijpbs.net/vol-3/issue-1/bio/P%20-%2052.pdf
37. Lakshmi BVS, Sudhakar M. Protective effect of zingiber officinale on Gentamicin-Induced nephrotoxicity in rats. International Journal of Pharmacology [Internet]. 15 de diciembre de 2009;6(1):58-62. Available in: https://doi.org/10.3923/ijp.2010.58.62
38. Agbafor KN, Nwaka AC, Dasofunjo K, Nnaemeka UM. Creatinine, urea and uric acid levels in albino rats treated with leaf extract of Canjanus Cajan (PIGEON PEA). ResearchGate [Internet]. 1 de enero de 2017; Available in: https://www.researchgate.net/publication/330025606_creatinine_urea_and_uric_acid_levels_in_albino_rats_treated_with_leaf_extract_of_canjanus_cajan_pigeon_pea
39. Shahrajabian MH, Sun W, Cheng Q. Tremendous health benefits and clinical aspects of Smilax China. African Journal of Pharmacy and Pharmacology [Internet]. 31 de octubre de 2019;13(16):253-8. Available in: https://doi.org/10.5897/ajpp2019.5070
40. Epure A, Pârvu A, Vlase L, Benedec D, Hanganu D, Vlase AM, et al. Polyphenolic compounds, antioxidant activity and nephroprotective properties of Romanian Taraxacum officinale. Farmacia [Internet]. 25 de febrero de 2022;70(1):47-53. Available in: https://doi.org/10.31925/farmacia.2022.1.7
41. Almundarij TI, Alharbi Y, Abdel-Rahman HA, Barakat H. Antioxidant activity, phenolic profile, and nephroprotective potential of anastatica hierochuntica ethanolic and aqueous extracts against CCL4-Induced nephrotoxicity in rats. Nutrients [Internet]. 26 de agosto de 2021;13(9):2973. Available in: https://doi.org/10.3390/nu13092973
42. Hu J, Liu Z, Zhang H. Omega-3 fatty acid supplementation as an Adjunctive therapy in the treatment of chronic kidney disease: A Meta-analysis. Clinics [Internet]. 1 de enero de 2017;72(1):58-64. Available in: https://doi.org/10.6061/clinics/2017(01)10
43. Xie L, Skrezek C, Wand H. Amino acids’ protective effects on experimental acute renal failure. Journal of Zhejiang University-SCIENCE A [Internet]. 2000; 1(2):212-7. Available in: https://doi.org/10.1631/bf02839244
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest with the data contained in the manuscript.
AUTHORSHIP CONTRIBUTION
Conceptualization: Soledispa PA, Mangas R.
Data curation: Soledispa PA, Choez I, Manzano P.
Formal analysis: Soledispa PA, Mangas R, Sarmiento G, Manzano P, Choez I.
Research: Soledispa PA, Mangas R, Sarmiento G, Manzano P, Choez I, Zavala B.
Methodology: Soledispa PA, Mangas R, Sarmiento G, Manzano P, Choez I.
Software: Manzano P, Choez I, Zavala B.
Supervision: Soledispa PA, Mangas R.
Validation: Soledispa PA, Mangas R.
Drafting - original draft: Soledispa PA, Sarmiento G, Zavala B.
Writing - proofreading and editing: Soledispa PA, Sarmiento G, Zavala B.