doi: 10.56294/saludcyt2023564
ORIGINAL
Comparison of radiation protection effects between epigallocatechin gallate and ascorbic acid
Comparación de los efectos de protección radiológica entre el galato de epigalocatequina y el ácido ascórbico
Tran Thi Nhan1 ![]() *, Youichirou Matuo2
*, Youichirou Matuo2![]() *,
Yoshinobu Izumi3
*,
Yoshinobu Izumi3![]() *,
Maradi Abdillah4
*,
Maradi Abdillah4 ![]() *, Lukas Wisnu Wicaksono4
*, Lukas Wisnu Wicaksono4![]() *,
Vuong Thu Bac5
*,
Vuong Thu Bac5 ![]() *
*
1Electric Power University, Vietnam.
2Division of Nuclear Safety Engineering, University of Fukui, Japan.
3Research Institute of Nuclear Engineering, University of Fukui, Japan.
4Indonesia Nuclear Energy Regulatory Agency, Indonesia.
5Vietnam Atomic Energy Institute, Vietnam.
Cite as: Nhan TT, Matuo Y, Izumi Y, Abdillah M, Wicaksono LW, Bac VT. Comparison of radiation protection effects between epigallocatechin gallate and ascorbic acid. Salud, Ciencia y Tecnología 2023; 3:564. https://doi.org/10.56294/saludcyt2023564.
Submitted: 20-08-2023 Revised: 14-10-2023 Accepted: 05-12-2023 Published: 06-12-2023
Editor: Dr.
William Castillo-González![]()
ABSTRACT
Ionizing radiation can originate from naturally occurring radiation sources on the earth or it can be from man-made sources. When interacting with cells and living organisms, ionizing radiation produces free radicals, impacting biological molecules such as proteins, lipids, and DNA in the cell nucleus and membrane, leading to cell death or causing cell mutations. Epigallocathecin gallate (EGCG) and ascorbic acid (AA) are well-known natural antioxidants that have been studied and applied as potential radical scavengers. In this study, the radiation protection effects in the presence of EGCG and AA via the scavenging process of free radicals (mainly hydroxyl radicals) were examined. Saccharomyces cerevisiae yeast cells were grown in YDP liquid medium containing yeast extract, peptone, and dextrose/glucose that supplemented with EGCG and AA at different concentrations. Then, the cell cultures were irradiated with both low (gamma) and high (helium ion beam) linear energy transfer (LET) radiations to evaluate the radiation effect on the survival of the yeast cell. Both of EGCG and AA play effectively important roles as radiation-protective agent for yeast cells and the effectiveness in radiation protection of EGCG and AA at the same concentration was almost the same between these two additives.
Keywords: Antioxidant; Radical scavenger; Epigallocatechin Gallate; Ascorbic Acid; Radiation.
RESUMEN
Las radiaciones ionizantes pueden proceder de fuentes de radiación naturales de la Tierra o de fuentes artificiales. Al interactuar con las células y los organismos vivos, las radiaciones ionizantes producen radicales libres que afectan a moléculas biológicas como las proteínas, los lípidos y el ADN del núcleo y la membrana celular, provocando la muerte de las células o causando mutaciones celulares. El galato de epigalocatequina (EGCG) y el ácido ascórbico (AA) son antioxidantes naturales bien conocidos que se han estudiado y aplicado como potenciales eliminadores de radicales. En este estudio, se examinaron los efectos de protección contra la radiación en presencia de EGCG y AA mediante el proceso de eliminación de radicales libres (principalmente radicales hidroxilo). Se cultivaron células de levadura Saccharomyces cerevisiae en un medio líquido YDP que contenía extracto de levadura, peptona y dextrosa/glucosa, complementado con EGCG y AA en diferentes concentraciones. A continuación, los cultivos celulares se irradiaron con radiaciones de transferencia de energía lineal (LET) bajas (gamma) y altas (haz de iones de helio) para evaluar el efecto de la radiación en la supervivencia de la célula de levadura.
Tanto el EGCG como el AA desempeñan eficazmente importantes funciones como agentes protectores de las células de levadura frente a la radiación, y la eficacia en la protección frente a la radiación del EGCG y el AA en la misma concentración fue casi la misma entre estos dos aditivos.
Palabras clave: Antioxidante; Captador de Radicales; Galato de Epigalocatequina; Ácido Ascórbico; Radiación.
INTRODUCCIÓN
Ionizing radiation emanates from both naturally occurring substances and artificial radiation sources, both of which pose risks to the human body. In case of a nuclear accident, a large amount of radioactive material can be released into the environment. When interacting with cells and living organisms, ionizing radiation generates free radicals, affecting biological molecules found in the cell nucleus such as proteins, lipids, and DNA, resulting in cell death or causing cellular mutations.(1) These free radicals may cause damages to DNA, including single-strand breaks (SSBs) in phosphodiester bonds or double-strand breaks (DSBs) on opposite or displaced sites, base damage, protein-DNA crosslinking and protein-protein crosslinking. Double-strand breaks (DSBs) cause more severe damage to cells because they are difficult to repair or the repair process may not be completed, leading to the appearance of mutations (abnormal cells, also known as cancer cells).(2,3) Furthermore, the results of ionizing radiation on biological organisms are often believed to be based on linear energy transfer (LET).(1) High LET radiation lead to more localized multiple DSBs. High LET radiation is capable of causing more double - strand breaks (DSB) of DNA in cells in comparison with low LET. The DSB causes more serious damage to cells because the damage of DSB is difficult to repair or the repair process is incomplete and mutations.(3) Also, the assessment of cell survival is one of the important indicators to study the biological effects of ionizing radiation.
Cell degeneration is a primary underlying cause of various diseases in the human body. In particular, cancer is closely associated with cellular degeneration, where mutated cells tend to be more active in producing compounds containing O−O single bonds. These diseases are the result of the overproduction of reactive oxygen species or reactive oxygen species (ROS) in the metabolic activities of the cells, one of which is caused by ionizing radiation. Cellular damage, including lipid peroxidation, DNA damage resulting in genetic miscommunication, protein oxidation, enzyme inactivation, and ultimately cell death, is caused by oxidative stress. The body of animals or humans often stores compounds with high antioxidant properties such as glutathione, vitamin E, and vitamin C ... When the content of antioxidants in the body decreases, the risk of cells will increase. Cells are oxidized and transformed by ROS. However, the adverse effects of ROS can be prevented by supplementing with a diet rich in foods that contain antioxidants (legumes, fresh vegetables, and fruits, etc.).(4,5) Natural antioxidants such as polyphenol compounds have the ability to reduce free radicals, prevent oxidation effectively and are often found in natural products such as green tea or sour fruits. AA is well known as a protective agent.(6,7) Green tea is widely used in Asian countries and is considered an antioxidant food.(8-11) The radioprotection ability of epigallocatechin gallate (EGCG) in green tea against gamma radiation in mice has also been studied.(12) In this study, EGCG and AA, natural antioxidants were used as radical scavengers to evaluate their protection against gamma radiation (low LET) and helium ion beam (high LET).
METHODS
Preparation of yeast cells
S. cerevisiae cells (RAD+) were cultured for 24 h at 30°C in liquid YPD medium without additives or supplemented with 300μM, 500μM and 1000μM EGCG or AA. After 24 hours of culture, the inoculum was inoculated into a Neubauer counting chamber to count the number of viable cells under the microscope at 400x magnification.
After the initial concentration was known, the yeast cell solutions were diluted in log phase to a concentration of 40 cells/ml, and every 5 ml of yeast cell solution was then filtered through a vacuum filter (Sterifil sterile system), Milipore; Billerica, MA) using 0.45 μm pore size nitrocellulose membranes of 47 mm diameter (Milipore®; Billerica, MA). Thus, the YPD medium is removed and the yeast cells remain on the membrane filter. The membrane contained 200 cells and was laid down in a 50 mm diameter dish and irradiated. At one dose point, 5 membranes were prepared for each group of antioxidant content.
Antioxidant compounds
EGCG and AA were purchased from Nagara Science Co., Ltd. (Japan) with purity greater than or equal to 99 %. To analyze the cell survival rate changes depending on the concentration of antioxidants, EGCG and AA were dissolved in YPD liquid medium with dissimilar molar concentrations.
Radiation sources
Two types of radiation, low LET (gamma rays) and high LET (helium ion) are set up to study the effects of radiation on yeast cells.
The gamma radiation source used in this study is Co-60. This radiation source belongs to the Institute of Scientific and Industrial Research (ISIR), Osaka University. The source activity under irradiation was 127,66TBq (LET 0,2 keV/μm). To achieve the required absorbed doses, the samples were placed at the appropriate distance from the radiation source (Co-60) and the exposure time was determined to be 30 minutes. Moreover, the heavy ion used in this study was Helium Ion with an energy of 220MeV (55MeV/u) and with a LET of 5,5 keV/μm at the Wakasa-Wan Energy Research Center.
Survival rate and protection ratio.
Cell survival was determined based on its ability to grow into a colony after culture on YPD. The number of yeast cells that survived irradiation with a given dose was determined by counting colonies growing on solid YPD medium.
The number of viable cells remaining after irradiation can be compared with the original cell count (non - irradiated) to get the survival rate (S), as shown in the formula below:
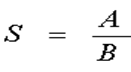 (1)
(1)
A: Colony number of irradiated cell group
B: Colony number of non-irradiated cell group
Radiation protection ratio (PR) given from EGCG and AA for each molarity at each dose, using the formula below:
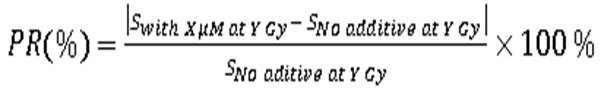 (2)
(2)
RESULTS
|
Table 1. Survival rate of yeast exposed to gamma-ray in medium containing (-)-epigallocatechin gallate (EGCG) and ascorbic acid (AA) with different concentrations |
|||||
|
Absorbed Dose (Gy) |
0 |
25 |
50 |
75 |
100 |
|
No additive |
1 |
0,641 |
0,593 |
0,446 |
0,361 |
|
EGCG 300 μM |
1 |
0,824 |
0,799 |
0,731 |
0,567 |
|
EGCG 500 μM |
1 |
0,997 |
0,920 |
0,704 |
0,531 |
|
EGCG 1000 μM |
1 |
0,868 |
0,837 |
0,788 |
0,5489 |
|
AA 300 μM |
1 |
0,938 |
0,709 |
0,749 |
0,620 |
|
AA 500 μM |
1 |
0,777 |
0,757 |
0,621 |
0,454 |
|
AA 1000 μM |
1 |
0,848 |
0,857 |
0,838 |
0,565 |
|
Table 2. Survival rate of yeast exposed to helium ion beam in medium containing (-)-epigallocatechingallate (EGCG) and ascorbic acid (AA) with different concentrations |
|||||
|
Absorbed Dose (Gy) |
0 |
25 |
50 |
75 |
100 |
|
No additive |
1 |
0,580 |
0,430 |
0,387 |
0,220 |
|
EGCG 300 μM |
1 |
0,847 |
0,822 |
0,751 |
0,582 |
|
EGCG 500 μM |
1 |
0,989 |
0,804 |
0,613 |
0,516 |
|
EGCG 1000 μM |
1 |
0,894 |
0,765 |
0,741 |
0,534 |
|
AA 300 μM |
1 |
0,970 |
0,879 |
0,795 |
0,596 |
|
AA 500 μM |
1 |
0,766 |
0,747 |
0,599 |
0,399 |
|
AA 1000 μM |
1 |
0,917 |
0,857 |
0,838 |
0,587 |
Tables 1 and 2 indicated that the addition of EGCG or AA significantly increased the survival rate of yeast cells after irradiation with both low LET (gamma ray) and high LET (helium ion beam) radiation. The results obtained proved that EGCG and AA play radiation protection of cells again ionizing radiation. Based on the data from tables 1 and 2, we calculated the protection ratio of cells of EGCG and AA according to the molar concentration to compare the antioxidant ability of the compounds. This comparison was depicted in figure 1 and figure 2.
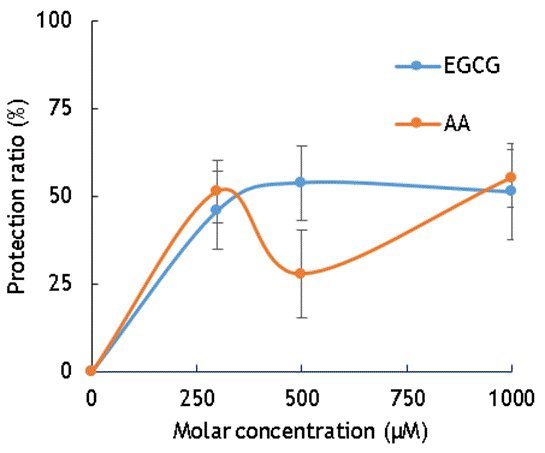
Figure 1. The protection ratio of EGCG and AA on cells irradiated with gamma rays
Figure 1 indicates the radiation protection of cells given for 300μM, 500μM, and 1000μM of EGCG and AA. The highest gamma irradiation protection on cells of AA is 51,3 % at 300µM and the number for EGCG is 53,8 % at 500µM. The data show that the radiation protections on cells irradiation by gamma rays are almost equal for AA and EGCG at 300µM and 1000µM. The protection ratio of EGCG against gamma irradiation is higher than AA at 500µM, however, the error bar at this molar concentration is so high.
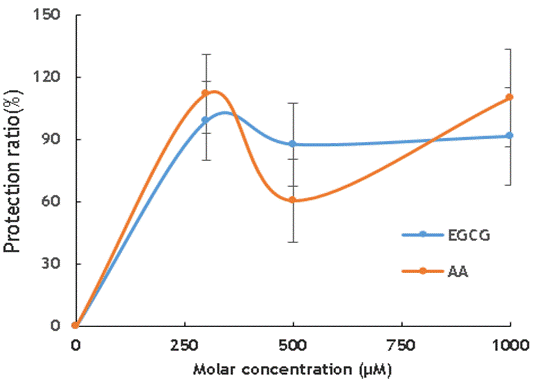
Figure 2. The protection ratio of EGCG and AA on cells irradiated with heliumion
The protective ability of EGCG and AA to cells against the effects of high LET radiation is more apparent. With a molar concentration of 300µM, the protection against the effects of helium beam ion is highest. At 500µM and 1000µM, the radiation protection effects of EGCG and AA are almost the same within experimental error.
The results obtained from figure 1 and figure 2 indicated that both EGCG and AA play radiation protection of cells and the protections are almost the same with low LET radiation and high LET radiation.
DISCUSSION
Previous research shows that EGCG is more active than AA in terms of free radical scavenging.(13-15) Jeffrey Johns and his colleague used the Electron donor index (Rd) and Electron acceptor index (Ra) to classify the free radical scavenging capabilities. The smaller the value of Rd, the easier electron can be donated by the compound. From his research, we can see that EGCG has a lower Rd score than AA, this means EGCG can easily donate their electrons to free radicals compared to AA. This makes EGCG a better compound to scavenge free radicals compared to AA.(16) The donor–acceptor map of some representative antioxidants is shown in figure 3.
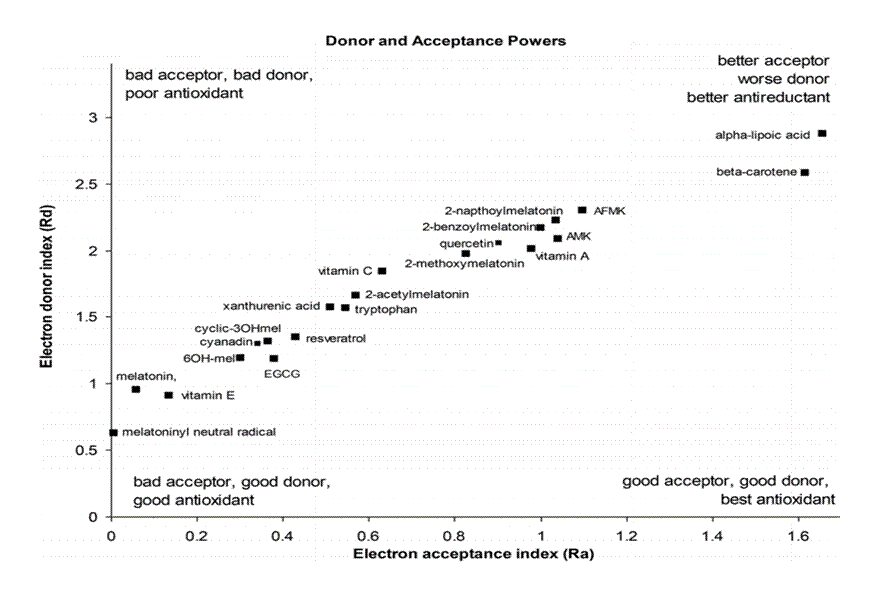
Figure 3. Donor–acceptor map of representative antioxidants(16)
Besides using Rd and Ra mapping, other researchers also investigated the scavenging capabilities of EGCG and AA using different methods. One of the methods is by measuring the speed of the reaction (rate constant) of EGCG and AA with ·OH radicals formed from water radiolysis due to the indirect effect of radiation, the most reactive free radical among reactive oxygen species. The rate constants were obtained as 4,62 x 1011 M-1 s-1 for EGCG,(17) and 1,2 x 1010 M-1s-1 for AA.(18) The rate constant is the change in concentration of reactants or products per unit of time. A higher rate constant indicates a greater change in the concentration of reactants or products per second. EGCG exhibits a higher rate constant (k) than AA, suggesting that EGCG may have the faster reaction or scavenging capabilities with. OH radicals compared to AA.
It was studied that the antioxidant activity can be explained throught the number of OH groups in the molecular structure of compounds. It has been shown that the more OH groups a compound contains in its molecular structure, the higher its antioxidant activity.(19)
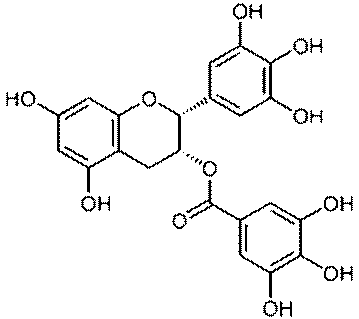
a.
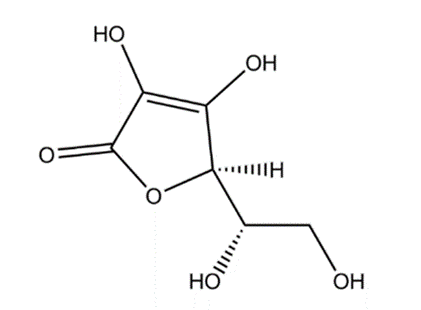
b.
Figure 4. Chemical structure of Radical scavenger. (a) Epigallocathechin gallate, (b) Ascorbic acid
In the molecular structure of EGCG there are 8 hydroxyl groups, while AA possesses only 4 hydroxyl groups in the molecular structure. It is the unequal number of OH groups present in the molecular structure that makes the free radical scavenging ability of EGCG is stronger than that of AA. EGCG possesses 3 extra hydroxyl groups at C-3' and C-4', and C-5'. The addition of a hydroxyl group at C-5' of the B ring also contributes to free radical scavenging activity, so EGCG has a stronger antioxidant capacity. In addition, in the molecular structure of EGCG, the C-3 site of the C-ring is linked to a gallate group via an ester linkage. The presence of the gallate group at the C-3 of the C ring plays an important role in the free radical scavenging capacity of EGCG.(19)
On another hand, other structural features influence the antioxidant activity. Concerning the radiation protection effect, it can be generally said that lower excitation energy of singlet excited state is advantageous. The excitation energy of the reactive intermediate species is efficiently transferred to the protective agent, and the greater the electron affinity, the more likely the electron transfer reaction from the anion radicals among the reactive intermediate species to the protective agent will occur, and resulting the more likely the anion radical of the protective agent will be formed, which is advantageous, moreover, the lower the ionization potential, the easier it is for a hole transfer reaction to occur from the cation radicals among the reactive intermediate species to the protective agent, which is advantageous because the cation radicals of the protective agent are likely to be generated.(20) Also, the higher the hydrogen-donating ability of the protective agent molecule, the higher the efficiency of detoxifying the OH radical and transforming it into a water molecule by donating hydrogen to the OH radical, which is advantageous. Yamina Boulmokh et al. reported the results of an experimental study aimed at clarifying the structure-activity relationship of antioxidants and their mechanisms of reaction.(20) In particular, in that report, the antioxidant activities of resveratrol (RSV), EGCG, EC, and AA were investigated using the DPPH method and FRAP assay. They also adopted density functional theory (DFT) methods for theoretical calculations, and considered 3 mechanisms of (i) hydrogen atom transfer (HAT), (ii) single electron transfer-proton transfer (SET-PT), and (iii) sequential proton loss electron transfer (SPLET). Their calculation results show that EGCG is a more potent antioxidant than EC and RSV. They also showed that EGCG has a very interesting ability to reduce Fe3+ ions, especially at very low concentrations. On the other hand, the DPPH free radical scavenging assay showed that the antiradical activity of EGCG was higher than that of RSV but lower than that of AA (Figure 5).
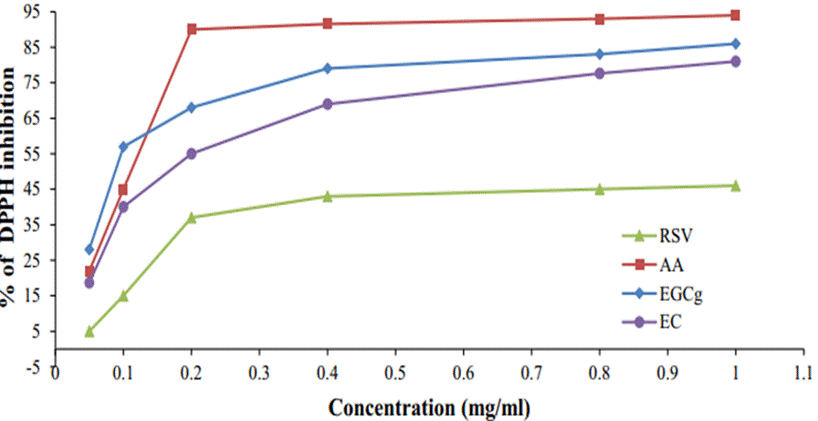
Figure 5. Percent inhibition of DPPH vs concentration of EGCg, EC, RSV, and AA. The results were studied by Yamina Boulmokh et al(21)
Therefore, in other words, multiple factors affect the efficiency of the radiation protection effect, and the totality of these factors determines the efficiency of the radiation protection effect. It can be understood that in our results shown in Figs 1 and 2, there are no significant difference within the experimental error was obtained.
CONCLUSIONS
The survival rate of cells and protection ratio were calculated when yeast cells were cultured in YDP medium and added EGCG and AA with different concentrations. Low LET (gamma ray) and high LET (helium ion beam) are used to study the effects of radiation on yeast cells. The results show that both EGCG and AA play radiation protection of cells and the antioxidant activities of EGCG and AA are almost the same.
BIBLIOGRAPHIC REFERENCES
1. International Atomic Energy Agency. Radiation Biology: A Handbook for Teachers and Students. TCS (Training Course Series), IAEA, Vienna, Austria, 2010.
2. Satish Balasaheb Nimsea, and Dilipkumar Palb. Free radicals, natural antioxidants, and their reaction mechanisms. The Royal Society of Chemistry. 2015; 5: 27986-28006. https://doi.org/10.1039/C4RA13315C
3. Francesca Ballarini. From DNA Radiation Damage to Cell Death: Theoretical Approaches. Journal of Nucleic Acids, 2010. doi:10.4061/2010/350608
4. Youichirou Matuo, Yoshinobu Izumi, Norihito Sato, Takayoshi Yamamoto, Kikuo Shimizu. Evaluation Of DNA Lesions Caused By High-LET Radiation Using The Polymerase Chain Reaction. Radiation Measurements. 2013; 55: 93-95. https://doi.org/10.1016/j.radmeas.2013.01.016
5. Dejian Huang, Boxin Ou and Ronald L. Prior. The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry. 2005; 53: 1841-1856. https://doi.org/10.1021/jf030723c
6. Sudha J. Devaki and Lali Raveendran. Vitamin C: Sources, Functions, Sensing and Analysis. Janeza Trdine 9, 51000 Rijeka, Croatia, 2017.
7. Buettner G R. The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, Α-Tocopherol, And Ascorbate. Archives of Biochemistry and Biophysics. 1993; 300: 535-543. https://doi.org/10.1006/abbi.1993.1074
8. Brahma N. Singh, Sharmila Shankar, And Rakesh K. Srivastava*. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem Pharmacol. 2011; 82(12): 1807–1821. https://doi.org/10.1016/j.bcp.2011.07.093
9. Fumio NANJO, Masao MORI, Keiichi GOTO, Yukihiko HARA. Radical Scavenging Activity of Tea Catechins and Their Related Compounds. Bioscience, Biotechnology, And Biochemistry. 1999; 63(9):1621-1623. https://doi.org/10.1271/bbb.63.1621
10. Qiong Guo, Baolu Zhao, Meifen Li, Shengrong Shen, Wenjuan Xin. Studies On Protective Mechanisms of Four Components of Green Tea Polyphenols Against Lipid Peroxidation in Synaptosomes. Biochimica Et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1998; 1394(2-3): 210-222. https://doi.org/10.1016/S0005-2760(96)00122-1
11. Mao-Jung Lee, Pius Maliakal, Laishun Chen, Xiaofeng Meng, Flordeliza Y. Bondoc, Saileta Prabhu, George Lambert, Sandra Mohr, And Chung S. Yang. Pharmacokinetics of Tea Catechins After Ingestion of Green Tea And (-)-Epigallocatechin-3-Gallate By Humans: Formation of Different Metabolites and Individual Variability. Cancer Epidemiology, Biomarkers & Prevention, American Association for Cancer Research. 2002; 11: 1025-1032.
12. Sheila A. Wiseman, Douglas A. Balentine, Balz Frei. Antioxidants in tea. Critical Reviews in Food Science and Nutrition. 2009; 37 (8): 705-718. https://doi.org/10.1080/10408399709527798
13. Michael Sauer, Paola Branduardi, Minoska Valli, And Danilo Porro. Production Of L-Ascorbic Acid By Metabolically Engineered Saccharomyces Cerevisiae And Zygosaccharomyces Bailii. Applied and Environmental Microbiology. 2004; 7(10): 6086-6091. https://doi.org/10.1128/AEM.70.10.6086-6091.2004
14. Yamina Boulmokh, Karima Belguidoum, Faiza Meddour, Habiba Amira-Guebailia. Investigation of antioxidant activity of epigallocatechin gallate and epicatechin as compared to resveratrol and ascorbic acid: experimental and theoretical insights. Structural Chemistry. 2021; 32: 1907–1923. https://doi.org/10.1007/s11224-021-01763-5
15. Silvia López-Burillo 1, Dun-Xian Tan, Juan C Mayo, Rosa M Sainz, Lucien C Manchester, Russel J Reiter. Melatonin, xanthurenic acid, resveratrol, EGCG, vitamin C and alpha-lipoic acid differentially reduce oxidative DNA damage induced by Fenton reagents: a study of their individual and synergistic actions. Journal of Pineal Research. 2003; 34: 269–277. doi: 10.1034/j.1600-079x.2003.00041.x.
16. Jeffrey R. Johnsa and James A. Platts. Theoretical Insight In to The Antioxidant Properties Of Melatonin And Derivatives. Organic and Biomolecular Chemistry. The Royal Society of Chemistry. 2014; 12: 7820-7827. DOI: 10.1039/C4OB01396D
17. Xianglin Shi, Jianping Ye, Stephen S. Leonard, Min Ding, Val Vallyathan, Vincent Castranova, Yon Rojanasakul, And Zigang Dong. Antioxidant Properties Of (-)-Epicatechin-3-Gallate And Its Inhibition Of Cr(Vi)-Induced DNA Damage And Cr(Iv)- Or TPA-Stimulated NF-Kb Activation. Molecular And Cellular Biochemistry. 2000; 206: 125–132. https://doi.org/10.1023/A:1007012403691
18. Leon M. Dorfman, Gerald E. Adams, Reactivity Of The Hydroxyl Radical In Aqueous Solution, National Standard Reference Data System, U.S. National Bureau Of Standards, 1973.
19. Lan-Sook Lee, Sang-Hee Kim, Young-Boong Kim and Young-Chan Kim. Quantitative Analysis of Major Constituents in Green Tea with Different Plucking Periods and Their Antioxidant Activity. Molecules. 2014; 19: 9173-9186. https://doi.org/10.3390/molecules19079173.
20. Shigehiko Tabuse, Yoshinobu Izumi, Takao Kojima, Yoichi Yoshida, Takahiro Kozawa, Miyako Miki, Seiichi Tagawa. Radiation Protection Effects By Addition Of Aromatic Compounds To N-Dodecane. Radiation Physics And Chemistry. 2001; 62: 179-187. https://doi.org/10.1016/S0969-806X(01)00436-4.
21. Chaparro-Montoya EE, Vera-Alcázar MM, Herrera-Córdova FB, Barahona-Sánchez JC. Utilización de microorganismos eficientes para la elaboración de compost a partir de residuos orgánicos. Sincretismo 2020;1.
22. Yamina Boulmokh, Karima Belguidoum, Faiza Meddour, Habiba Amira-Guebailia. Investigation of antioxidant activity of epigallocatechin gallate and epicatechin as compared to resveratrol and ascorbic acid: experimental and theoretical insights. Structural Chemistry. 2021; 32: 1907–1923. https://doi.org/10.1007/s11224-021-01763-5.
FINANCING
The authors received financing for the development of this research from FuKui University, Japan.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Youichirou MATUO, Yoshinobu IZUMI
Data curation: TRAN Thi Nhan, Maradi Abdillah, Lukas Wisnu Wicaksono.
Formal analysis: TRAN Thi Nhan, Maradi Abdillah
Acquisition of funds: Youichirou MATUO, Yoshinobu IZUMI
Research: TRAN Thi Nhan, Maradi Abdillah
Methodology: TRAN Thi Nhan, Youichirou MATUO, Yoshinobu IZUMI
Project management: Yoshinobu IZUMI
Resources: TRAN Thi Nhan, Youichirou MATUO, Yoshinobu IZUMI
Supervision: Yoshinobu IZUMI
Validation: TRAN Thi Nhan
Display: TRAN Thi Nhan
Drafting - original draft: TRAN Thi Nhan, Youichirou MATUO, Yoshinobu IZUMI, VUONG Thu Bac
Writing - proofreading and editing: TRAN Thi Nhan, Youichirou MATUO, Yoshinobu IZUMI