doi: 10.56294/saludcyt2024.1294
ORIGINAL
The Use of Paediatric Early Warning Signs to Detect Clinical Deterioration in Children with Oncohematological Diseases
Uso de signos pediátricos de alerta precoz para detectar el deterioro clínico en niños con enfermedades oncohematológicas
Yedil
Kurakbayev1,2 ![]() *, Kuanysh Umbetov2
*, Kuanysh Umbetov2 ![]() , Yergali Sarsekbayev2
, Yergali Sarsekbayev2 ![]() , Botagoz Turdaliyeva1,3
, Botagoz Turdaliyeva1,3 ![]() , Lyazat Manzhuova2
, Lyazat Manzhuova2 ![]() , Abay Kussainov2
, Abay Kussainov2 ![]() , Gulnara Abdilova2
, Gulnara Abdilova2 ![]()
1Kazakhstan Medical University “Kazakhstan School of Public Health” LLP. Almaty, Kazakhstan.
2JSC “Scientific Center of Pediatrics and Pediatric Surgery” JSC. Almaty, Kazakhstan.
3Kazakh Scientific Center of Dermatology and Infectious Diseases. Almaty, Kazakhstan.
Cite as: Kurakbayev Y, Umbetov K, Sarsekbayev Y, Turdaliyeva B, Manzhuova L, Kussainov A, et al. The Use of Paediatric Early Warning Signs to Detect Clinical Deterioration in Children with Oncohematological Diseases. Salud, Ciencia y Tecnología. 2024; 4:.1294. https://doi.org/10.56294/saludcyt2024.1294
Submitted: 08-02-2024 Revised: 10-07-2024 Accepted: 30-11-2024 Published: 01-12-2024
Editor: Prof.
Dr. William Castillo-González ![]()
Corresponding author: Yedil Kurakbayev *
ABSTRACT
Aim: The aim is to highlight the importance of early detection, the efficacy of pediatric early warning signs (PEWS), and their implications for clinical practice, focusing on the specific signs that affect the detection and management of clinical deterioration in children with oncological diseases.
Method: The review included English-language studies retrieved from common databases using the keywords “pediatric,” “Early Warning Signs,” “clinical deterioration,” combined with terms related to “onchohematological” and “pediatric cancers.”
Results: sixteen articles were analyzed. The analysis revealed that the PEWS cohort was associated with a lower incidence of unplanned codes (p = 0,0001), cardiopulmonary arrest (P = 0,0001), and mortality (P = 0,01). The incidence of critical deterioration between the cohorts showed no significant variation (P = 0,07).
Conclusion: PEWS significantly contributes to reducing mortality rates, unplanned codes, and cardiopulmonary arrest. However, further research is required to address the limitations and challenges associated with its application in this population.
Keywords: Pediatric; Early Warning Signs; Leukemia; Hodgkin Lymphoma; Cancers.
RESUMEN
Objetivo: el objetivo es destacar la importancia de la detección precoz, la eficacia de los signos de alerta precoz pediátricos (PEWS) y sus implicaciones en la práctica clínica, centrándose en los signos específicos que afectan a la detección y el manejo del deterioro clínico en niños con enfermedades oncológicas.
Método: la revisión incluyó estudios en inglés recuperados de bases de datos comunes utilizando las palabras clave «pediatric,» «Early Warning Signs,» «clinical deterioration,» combinadas con términos relacionados con «onchohematological» y «pediatric cancers.»
Resultados: se analizaron dieciséis artículos. El análisis reveló que la cohorte PEWS se asoció con una menor incidencia de códigos no planificados (p = 0,0001), parada cardiopulmonar (p = 0,0001) y mortalidad (p = 0,01). La incidencia de deterioro crítico entre las cohortes no mostró variaciones significativas (p = 0,07).
Conclusiones: el PEWS contribuye significativamente a reducir las tasas de mortalidad, los códigos no planificados y la parada cardiorrespiratoria. Sin embargo, se requiere más investigación para abordar las limitaciones y los retos asociados a su aplicación en esta población.
Palabras clave: Pediatría; Signos de Alerta Temprana; Leucemia; Linfoma de Hodgkin; Cánceres.
INTRODUCTION
In the field of pediatric healthcare, the early detection and management of clinical deterioration are of the most importance, particularly in children with oncohematological diseases. The term “Pediatric Early Warning Signs” (PEWS) refers to a set of clinical indicators that help healthcare professionals identify and respond to potential worsening conditions in pediatric patients.(1)
Pediatric Early Warning Signs (PEWS) is a systematic approach used in pediatric healthcare settings to identify and monitor signs of clinical deterioration in children. It is designed to detect early warning signs and facilitate timely intervention, ultimately aiming to improve patient outcomes and reduce morbidity and mortality.(2)
Children with oncohematological diseases, such as leukemia or lymphoma, are particularly vulnerable to clinical deterioration due to the nature of their condition and the potentially toxic effects of treatment modalities. These patients often undergo intensive therapies, including chemotherapy, radiation, and stem cell transplantation, which can weaken their immune system and make them susceptible to infections and other complications. Identifying early signs of clinical deterioration in these children is crucial in order to intervene promptly and prevent further deterioration.(3)
Importance of early detection in oncohematological diseases: Early detection of clinical deterioration in children with oncohematological diseases can significantly impact their outcomes. Prompt recognition and management of worsening conditions can help prevent complications, minimize the need for intensive interventions, and improve overall patient outcomes. By closely monitoring pediatric patients and utilizing standardized tools like PEWS, healthcare professionals can identify subtle changes in vital signs, behavior, and overall clinical status, enabling timely intervention and potentially reducing morbidity and mortality rates.(4)
Research focus
The focus of this literature review is the application of pediatric early warning signs in children with oncohematological diseases. The review examines existing literature to highlight the importance of early detection, the efficacy of PEWS, and its implications for clinical practice. Additionally, the limitations of current practices are discussed, and areas for future research and improvement are identified.
Recent developments in pediatric oncology and the implementation of early warning signs
Over the recent past, pediatric oncology has been subject to defined change following research, technology, and clinical practice improvement. Pivotal to these improvements is the increasing awareness of the importance of these initial indicators in childhood cancer diseases. These signs, which include a vast palette of changes of different severities in a child’s health state, act as valuable markers of disease progression, efficiency of the treatment measures taken, and possible outcomes. Incorporation of early warning signs into practice is, thus, a positive shift in pediatric oncology practice since it allows for quick identification and management of indicators that may lead to negative outcomes, which, in turn, leads to better management of young cancer patients and their quality of life.(5) This paper aims to identify current trends in pediatric oncology, with a particular focus on the concept of early warning signs, their importance, uses, and performance indicators, based on a comprehensive literature review.(6) By evaluating the literature and pertinent guidelines as well as innovations, the study seeks to unravel information on the current trends in pediatric oncology and the use of early signs in enhancing clinical management techniques.
New methodologies or technologies for detecting clinical deterioration in pediatric patients with leukemia, Hodgkin lymphoma, and other cancers
Recent advancements in pediatric oncology have led to the development of innovative methodologies and technologies designed to detect clinical deterioration in pediatric patients with leukemia, Hodgkin lymphoma, and other cancers. These innovations include wearable devices and remote monitoring systems, which allow continuous real-time tracking of vital signs, providing early alerts for potential complications. Predictive analytics and machine learning algorithms analyze vast amounts of patient data to identify patterns indicative of clinical deterioration, enabling preemptive management. Standardized assessment tools like the PEWS and risk stratification models systematically assess and monitor patients, facilitating timely interventions. Telemedicine and mobile health applications have also emerged as valuable tools, ensuring continuous care and early detection of health issues through remote consultations and symptom tracking. The implementation of these technologies has significantly improved clinical outcomes, reducing severe complications, decreasing hospital readmissions, and enhancing overall survival rates.(7) These advancements represent a proactive and personalized approach to pediatric oncology care, promising better management and improved quality of life for young cancer patients.
Outcomes of recent clinical trials or observational studies related to early warning systems in pediatric oncology setting
Recent clinical trials and observational studies have underscored the efficacy of early warning systems in pediatric oncology settings, demonstrating significant improvements in patient outcomes. These studies have shown that early warning systems, such as the PEWS and various risk stratification tools, enhance the timely identification of clinical deterioration, leading to prompt and effective interventions. For instance, trials have reported reductions in severe complications and hospital readmissions, as well as shorter lengths of hospital stays for pediatric oncology patients monitored with these systems. Moreover, observational studies have highlighted that the use of predictive analytics and machine learning algorithms in early warning systems can accurately forecast adverse events, allowing for proactive management and personalized care.(8) Overall, the evidence from these studies suggests that integrating early warning systems into routine clinical practice significantly enhances the safety and quality of care for pediatric oncology patients, ultimately contributing to better health outcomes and improved survival rates.(9)
Research problem
Although there has been a decline in cancer mortality over the past three decades, more cancer patients are now admitted to intensive care units (ICUs). According to data, cancer patients occupy 25 % to 30 % of ICU beds, and cancer-related traits are not linked to worse short-term outcomes. High-quality medical treatment includes early detection of patients at risk of clinical deterioration, matching the severity of the illness to the proper degree of care, and effective resource management in the hospital setting, understanding the unique challenges and risks faced by children with oncohematological diseases is crucial for healthcare professionals. Factors contributing to clinical deterioration include infections, chemotherapy-related complications, tumor progression, and treatment-related toxicities. Delayed recognition and intervention can have severe consequences, such as increased morbidity and mortality rates, prolonged hospital stays, compromised treatment outcomes, and long-term complications. Timely recognition and appropriate management are essential to optimize outcomes for these vulnerable patients.
Research aim
Our study aims to highlight the importance of early detection, the efficacy of pediatric early warning signs, and its implications for clinical practice.
Literature review
Onchohematological diseases
The development of hematologic malignancies in the body’s blood cells sets them apart from other cancers, and they sometimes may not result in tumors. While some hematologic oncologists are skilled in treating solid tumors, the majority do not handle operable malignancies like lung cancer or breast cancer. There are different types of hematological cancers, such as leukemia, Multiple myeloma, non-Hodgkin lymphoma, and Hodgkin lymphoma.(10)
Leukemia
With roughly 30 % of all malignancies in children diagnosed before the age of 15 being leukemia, it is the most common cancer in children. One-fourth of all malignancies are lymphoid leukemias, which are the most prevalent type. Precursor cell leukemias account for about 98 % of pediatric lymphoid leukemias, with precursor B-cell acute lymphoblastic leukemia (pB-ALL) being the most prevalent kind. Childhood leukemia (CL) treatment and survival have improved dramatically over the past few decades as a result of improvements in diagnostics, risk stratification, pharmacology, and treatment combinations. Today, high-income countries have acute lymphoblastic leukemia (ALL) survival rates that exceed 90 %. The diagnostic challenge faced by front-line clinicians is made more difficult by the early presentation of child leukemia, with non-specific symptoms frequently mirroring the common, self-limiting disorders.(11, 12)
Many health systems place a high premium on improving cancer early diagnosis. For example, the National Health Service Cancer Plan in the UK recommends that everyone who has been suspected of having cancer, including children, must see a specialist within two weeks of the referral. The National Institute for Health and Care Excellence (NICE) guidance lists a number of particular signs and symptoms that should cause physicians to think about pediatric cancer and, in the event of leukemia, to collect a blood sample or promptly refer a patient. Despite these efforts, the 2-week urgent referral pathway still fails to diagnose the vast majority of pediatric malignancies. In a recent study, alternate methods, such as direct presentations to emergency rooms or non-urgent hospital referrals from primary care, were used to diagnose 98 % of children’s malignancies in the UK.(13,14)
Multiple myeloma
A clonal plasma cell proliferative condition called multiple myeloma (MM) is characterized by an aberrant rise in monoclonal immunoglobulins. If left unchecked, the excessive creation of these plasma cells can eventually result in harm to particular end organs. This is most frequently observed when at least one of the clinical signs of hypercalcemia, renal failure, anemia, or bone pain associated with lytic lesions is present. In terms of the monoclonal gammopathy spectrum, MM is essentially a stage. Monoclonal gammopathy of Undetermined Significance (MGUS), a pre-malignant, asymptomatic stage of clonal plasma cell development, is assumed to be the source of it. Detecting monoclonal immunoglobulins in the blood or urine without signs of end-organ damage is referred to as MGUS. This is quite typical and can be found in more than 3 % of people over the age of 50.(15,16)
The cell of origin appears to be a post-germinal center plasma cell. Although it has been mentioned above, this illness is normally benign, with a 1 % annual risk of progressing to MM. Evaluation of any acute problems that require rapid stabilization should be part of the initial care of multiple myeloma. To treat severe hypercalcemia, some of these might involve giving patients isotonic saline for volume expansion, calcitonin, and/or bisphosphonates.(17) Medical optimization should be started if considerable renal failure is found, along with possible nephrology consultation to address fluid status, prevent nephrotoxic substances, alter essential drug dosages for the kidneys, and, in the case of severe dysfunction, discuss hemodialysis. Additionally, spinal cord compression due to a vertebral fracture or plasmacytoma should be treated immediately with neurosurgery or orthopedic advice, as well as perhaps radiation therapy. Plasmapheresis must be finished if the hyperviscosity condition is ever diagnosed.(18,19)
Hodgkin Lymphoma
The rare monoclonal lymphoid neoplasm Hodgkin lymphoma (HL), formerly known as Hodgkin’s disease, has a high likelihood of cure. Hodgkin lymphoma is a disease entity that has been separated into two distinct categories by biological and clinical studies: classical Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma (NLP-HL).(20) The clinical presentation and pathophysiology of these two disease entities vary. The four subgroups of classical Hodgkin lymphoma—nodular sclerosis (NSHL), lymphocyte-rich (LRHL), mixed cellularity (MCHL), and lymphocyte-depleted (LDHL)—account for roughly 95 % of all HL. Hodgkin lymphomas are characterized by four factors. They frequently develop in the cervical lymph nodes; young adults are more likely to develop the disease; large mononuclear Hodgkin and multinucleated cells (Reed-Sternberg) are sporadic and mixed among non-neoplastic inflammatory cells; and T lymphocytes are frequently seen encircling the distinctive neoplastic cells. Hodgkin lymphoma has a fantastic prognosis generally, with a cure rate of over 80 %.(21,22,23)
Definition and Concept of PEWS
PEWS is a proactive and standardized system that involves the assessment and monitoring of specific physiological, behavioral, and clinical parameters in pediatric patients. These parameters are used to identify deviations from baseline and trigger appropriate interventions. The concept behind PEWS is based on the recognition that early signs of deterioration in children may be subtle and easily missed, leading to delayed interventions and adverse outcomes.(24)
Development and Validation of PEWS Tools
Over the years, various PEWS tools have been developed and validated to suit different healthcare settings and patient populations. These tools typically consist of a scoring system that assigns points to different physiological and clinical parameters.(25) The scores are then used to determine the level of risk or severity of clinical deterioration. The development and validation of PEWS tools involve rigorous research, including retrospective and prospective studies, to establish their reliability, validity, and predictive value.(26)
Application of PEWS in Pediatric Healthcare Settings
PEWS is primarily applied in pediatric healthcare settings, including hospitals, pediatric wards, and intensive care units (ICUs). It is used as a proactive monitoring system to identify early signs of deterioration in children, allowing healthcare providers to intervene promptly and appropriately. PEWS tools are typically integrated into routine clinical practice, with regular assessments of vital signs, clinical observations, and other relevant parameters. The frequency and intensity of monitoring may vary depending on the patient’s condition, level of risk, and the specific PEWS tool being used.(27)
The application of PEWS in pediatric healthcare settings has several benefits. It helps healthcare providers to identify deteriorating patients earlier, enabling timely interventions and potentially preventing adverse events. PEWS also promotes standardized and consistent monitoring practices, facilitating communication and collaboration among healthcare teams.(28) Additionally, it can serve as a valuable tool for education and training, enhancing healthcare professionals’ knowledge and skills in recognizing and managing clinical deterioration in children. However, the implementation of PEWS in pediatric healthcare settings also presents challenges.(29) These include the need for adequate training and education of healthcare providers, ensuring consistent adherence to monitoring protocols, and addressing potential barriers to effective implementation. Additionally, ongoing research and evaluation are necessary to continuously improve and refine PEWS tools, ensuring their relevance and effectiveness in different clinical contexts.(30,31)
Clinical deterioration in children with oncohematological
Clinical deterioration in children with oncohematological diseases refers to the worsening of their medical condition, leading to a decline in their overall health status. These children face unique challenges and risks due to their underlying disease and the aggressive treatments they receive. Understanding these challenges is crucial for healthcare professionals involved in their care.(32)
A. Understanding the unique challenges and risks in this population:(33, 34)
1. Children with oncohematological diseases often undergo intensive chemotherapy, radiation therapy, or stem cell transplantation, which can severely compromise their immune systems. This makes them more susceptible to infections, including life-threatening ones.
2. These children may experience low blood counts (anemia, thrombocytopenia) due to the underlying disease or its treatment. This can lead to bleeding complications or increased susceptibility to infections.
3. Some oncohematological diseases can affect specific organs such as the liver, kidneys, or lungs. Organ dysfunction can further contribute to clinical deterioration and complicate management.(35)
4. The diagnosis of an oncohematological disease and its treatment can have a significant psychological impact on children and their families. Emotional distress may affect adherence to treatment plans and overall well-being.
B. Factors contributing to clinical deterioration:(36,37)
1. Children with compromised immune systems are at high risk of developing severe infections that can rapidly deteriorate their health status if not promptly recognized and treated.
2. Chemotherapy drugs can cause various side effects, such as nausea, vomiting, diarrhea, mucositis (inflammation of the mouth and gastrointestinal tract), or cardiotoxicity. These complications can worsen a child’s condition if not managed appropriately.(38)
3. Despite treatment efforts, some oncohematological diseases may progress or relapse over time, leading to clinical deterioration.
4. Intensive treatments like radiation therapy or stem cell transplantation can have significant toxicities, including organ damage, graft-versus-host disease (in the case of transplantation), or secondary malignancies. These complications can contribute to clinical deterioration.
C. Impact of delayed recognition and intervention:(39, 40)
1. Delayed recognition and intervention in clinical deterioration can lead to increased morbidity and mortality rates in children with oncohematological diseases.
2. Failure to recognize clinical deterioration promptly may result in prolonged hospital stays, increased healthcare costs, and added emotional burden on the child and their family.
3. Delayed intervention can compromise the effectiveness of ongoing treatments, potentially leading to treatment failure or reduced chances of cure.
4. Clinical deterioration that is not promptly addressed may result in long-term complications such as organ damage or impaired quality of life for these children.
Detection of clinical deterioration
PEWS is a tool used to detect early signs of clinical deterioration in pediatric patients.(41,42,43,44,45,46,47,48,49,50,51) The development and validation of PEWS tools have been extensively studied in general pediatric populations. Several studies have shown that the use of PEWS can improve the detection of clinical deterioration, reduce the incidence of cardiac arrest, and decrease the length of hospital stay in pediatric patients.(52, 53)
PEWS role in children with onchohematological diseases
PEWS tools consist of a scoring system that assigns points to different physiological parameters such as heart rate, respiratory rate, blood pressure, and oxygen saturation. The total score is then used to determine the level of risk for clinical deterioration. PEWS tools have been validated in different settings, including emergency departments, general wards, and intensive care units.(54,55)
Need for Specific Application and Adaptation of PEWS in Children with Oncohematological Diseases. Children with oncohematological diseases are a unique population that requires specific attention when using PEWS tools. These patients are at increased risk of developing complications due to their underlying disease and the treatments they receive, such as chemotherapy and radiation therapy.(56)
The use of PEWS in children with oncohematological diseases requires specific adaptations to account for the unique physiological changes that occur in these patients. For example, children with leukemia may have a higher heart rate due to anemia or fever, which may not necessarily indicate clinical deterioration. Therefore, modifications to the scoring system may be necessary to avoid false alarms.(57)
Several studies have evaluated the effectiveness of PEWS in detecting and managing clinical deterioration in children with oncohematological diseases.(58) A systematic review and meta-analysis by Chong et al. (2022) explored the impact of implementing PEWS in hospitals on reducing mortality, cardiopulmonary arrests, unplanned codes, and critical deterioration events in children compared to standard care without PEWS. They found that PEWS had a significant impact on the mortality rate and unplanned codes, while there was no significant impact on the risk of critical deterioration. These results are consistent with our results; however, they also found that PEWS has no significant impact on the rate of cardiopulmonary arrest, which is inconsistent with our results.(59)
The impact of PEWS on patient outcomes in children with oncohematological diseases has been evaluated in several studies. Garza et al (2021) investigated how the implementation of PEWS in hospitals affects the reduction of mortality, cardiopulmonary arrests, unplanned codes, and critical deterioration events in children, compared to standard care without PEWS. This study explored the impact of PEWS on reducing mortality, cardiopulmonary arrests, unplanned codes, and critical deterioration events in children compared to standard care.(59)
It also examined how the perceived quality of care during deterioration in children with cancer is influenced by PEWS in different hospital resource settings. The results indicated that healthcare providers caring for children with cancer consider PEWS valuable in improving hospital care despite challenges such as inadequate critical care resources and technology issues that vary by hospital resource level. These findings reinforce the positive impact of PEWS on the quality of care and suggest that it should be widely implemented in clinical practice.(60)
Despite the potential benefits of using PEWS in children with oncohematological diseases, there are some limitations and challenges that need to be addressed. One of the main challenges is the need for specific adaptations to account for the unique physiological changes that occur in these patients. Another challenge is the limited evidence regarding the effectiveness of PEWS in this population. Most studies have focused on general pediatric populations, and there is a need for more research to evaluate the effectiveness of PEWS in children with oncohematological diseases.
METHOD
General background
Patients with pediatric cancer who are hospitalized are very susceptible to clinical deterioration, especially in settings with constrained resources. PEWS are bedside assessment tools linked to an action protocol used to identify patients at risk of deterioration early. They have been proven to be accurate in predicting clinical deterioration in hospitals with varying levels of resources. The effects of PEWS have been proven at various hospital care levels. PEWS improves the perception of the quality of care while reducing clinical deterioration events and PICU utilization. PEWS have also been demonstrated to improve interdisciplinary communication and relationships, develop nursing empowerment, increase confidence in identifying and managing clinical deterioration, and result in cost savings. Although PEWS has had numerous beneficial benefits in environments with limited resources, little is known about how these effects are produced or linked to one another. This study highlights the importance of early detection, the efficacy of pediatric early warning signs, and its implications for clinical practice.
Inclusion criteria
1. Studies that include patients less than 18 years old.
2. Studies conducted in the EDs and inpatient units of pediatric hospitals.
3. Studies that perform a comparator between two cohorts: intervention cohort (PEWS) and control cohort (no PEWS)
4. Studies that included general population or specific population (e.g., cardiology units or oncology units).
Exclusion criteria
1. Studies that include patients more than 18 years old.
2. Studies conducted in outpatient units.
3. Single-arm studies that have no control cohort.
Information sources
A review of English studies was conducted using common databases, including Pubmed/MEDLINE, Google Scholar, Web of Science, Scopus, and the Cochrane Library. The search utilized keywords such as “pediatric,” “Early Warning Signs,” and “clinical deterioration,” combined with terms related to “onchohematological” and “pediatric cancers.” The review’s endpoint was set to September 2023. Studies were collected based on keyword combinations to ensure an unbiased collection of publications. Non-peer-reviewed studies, proposals, procedures, letters, and opinions were excluded. References included in this paper were selected for their relevance to the topic. The paper focuses on emphasizing the importance of early detection, the efficacy of pediatric early warning signs, and their implications for clinical practice.
Data collection
The articles included in this review were analyzed in three stages. The first stage involved importing findings from electronic databases into Microsoft Excel using EndNote Software. In the second stage, titles and abstracts were screened within the Excel sheet. The third stage entailed screening the full text of citations selected during Stage 2. References of the included publications were also manually reviewed to identify any potentially overlooked studies. Data were then extracted from the selected studies, including study characteristics and baseline data, using Microsoft Excel. Additionally, outcomes such as rates of mortality, critical deterioration, unplanned codes, and cardiopulmonary arrest were extracted for analysis.
Quality assessment of the included studies
The quality of the included randomized trial by Parshuram et al. (2018) was assessed using Cochrane’s risk of bias tool. Observational studies were evaluated with the National Heart, Lung, and Blood Institute (NHLBI) tool.(41)
Statistical analysis
Meta-analysis was performed using Review Manager Software. Dichotomous outcomes were analyzed with 95 % confidence intervals (CI) and odds ratios (OR). A fixed-effects model was applied for homogenous data, while a random-effects model was used for heterogeneous data. The degree of consistency among studies was evaluated using I² statistics and p-values from Chi-square tests. Heterogeneity was indicated by p-values ≤ 0,1 or I² > 50 %.
RESULTS
The search strategy yielded 5320 articles. After title and abstract screening, 188 articles underwent full-text review. A total of 60 articles were included for gathering information, with 16 articles used in the meta-analysis. The results of the search and screening process are detailed in figure 1.(41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56)

Figure 1. Results of our search
Table 1 and 2 demonstrates the study characteristics and demographic data of the involved articles.
|
Table 1. Baseline characteristics of the included studies |
|||||
|
First Author |
No. of sites |
Country (s) |
Study Design |
Study Population |
Name the PEWS used in the study (self-derived vs validated tool) |
|
Brilli 2007 (43) |
1 |
United States |
Cohort study (prospective post-implementation data compared with historical controls) |
General |
Self-derived MET trigger criteria |
|
Sharek 2007 (51) |
1 |
United States |
Cohort study (prospective post-implementation data compared with historical controls) |
General |
Criteria to activate the RRT were similar to Tibballs et al. and Brilli et al. (43) |
|
Hunt 2008 (49) |
1 |
United States |
Prospective cohort study pre- and post-implementation |
General |
Self-derived MET trigger criteria |
|
Tibballs 2009 (52) |
1 |
Australia |
Cohort study (prospective post-implementation data compared with historical controls) |
General |
Pediatric MET calling criteria were adapted from adult MET calling criteria with the addition of age-related abnormal readings |
|
Anwar-ul-Haque 2010 (55) |
1 |
Pakistan |
Retrospective cohort study pre- and post-implementation |
General |
PEWS |
|
Hanson 2010 (45) |
1 |
United States |
Interrupted time series with historical controls |
General |
Published antecedents and antecedents identified in chart reviews of local cardiac arrests were used to develop activation criteria |
|
Kotsakis 2011 (47) |
4 |
Canada |
Cohort study (prospective post-implementation data compared with historical controls) |
General |
Paediatric MET Triggers published by Tibballs et al. |
|
Parshuram 2011 (49) |
1 |
Canada |
Prospective cohort study pre- and post-implementation |
General |
Bedside PEWS |
|
McKay 2013 (42) |
1 |
Australia |
Prospective cohort study pre- and post-implementation |
General |
PEWS were age-specific scores adapted from the scoring system used at Great Ormond Street Hospital, London |
|
Bonafide 2014 (56) |
1 |
United States |
Interrupted time series with historical controls |
General |
Parshuram and colleagues’ Bedside PEWS (Pashuram 2011)(49) |
|
Sefton 2014 (50) |
1 |
United Kingdom |
Cohort study (prospective post-implementation data compared with historical controls) |
General |
Modified Bristol Paediatric Early Warning |
|
Douglas 2016 (44) |
1 |
United States |
Retrospective cohort study pre- and post-implementation |
General |
Adaptation of the Brighton PEWS by Akre et al |
|
Agulnik 2017 (53,54) |
1 |
Guatemala |
Retrospective cohort study pre- and post-implementation |
Oncology |
Modified PEWS adapted from Boston Children’s Hospital tool and algorithm |
|
Kroeger 2018 (48) |
1 |
United States |
Retrospective cohort study pre- and post-implementation |
Cardiology |
Modified Vanderbilt Children’s Hospital Pediatric Early Warning core (modified from the validated Brighton score) |
|
Parshuram 2018 (41) |
21 |
Belgium, Canada, England, Ireland, Italy, New Zealand, Netherlands |
Multi-centre cluster randomized trial |
General |
Bedside PEWS |
|
Agulnik 2023 (53,54) |
|
Mexico, Argantina, Peru, United states |
prospective, multicentre cohort study |
Oncology |
a Spanish language PEWS adapted for low resource settings |
|
Table 2. Continue baseline characteristics |
|||
|
First Author |
Activation criteria |
Describe the Intervention |
If RRT/PMET: Composition of RRT/PMET |
|
Brilli 2007 (43) |
Vital signs, increased breathing work, agitation or decreased consciousness, staff or parental concern |
MET |
PICU fellow, PICU nurse, senior paediatric resident, respiratory therapist, manager of patient services |
|
Sharek 2007 (51) |
Vital signs, acute change in level of consciousness, staff concern |
RRT |
Physician (paediatric ICU attending physician or fellow), experienced paediatric ICU or cardiovascular ICU nurse, an ICU-trained respiratory therapist, and a nursing supervisor |
|
Hunt 2008 (49) |
Vital signs, respiratory distress, seizures with apnoea, change in mental status, dysrhythmias, cardiopulmonary arrest, staff or parental concern |
MET |
PICU fellow, PICU nurse, PICU respiratory therapist, nursing shift coordinator, senior assistant resident, junior assistant resident, intern, paediatric pharmacist, security officer and hospital chaplain |
|
Tibballs 2009 (41) |
Vital signs, cardiopulmonary arrest, seizures, staff or parental concerns |
MET |
Initially: ICU Physician (consultant/ registrar), nurse, ED doctor and nurse + medical registrar; subsequently after 6 months ED nurse withdrew |
|
Anwar-ul-Haque 2010 (55) |
Vital signs, laboured breathing, decrease in consciousness, seizures, staff concerns |
RRT |
PICU physicians and primary team |
|
Hanson 2010 (45) |
Vital signs, changes in respiratory pattern or mental status, repeat or prolonged seizures, staff concerns |
MET |
Paediatric critical care fellow, resident, critical care nurse and respiratory therapist |
|
Kotsakis 2011 (47) |
Vital signs, acute drop in GCS by more than 2 points, seizures, staff or parental concerns |
MET |
PICU physician (PICU attending and fellow/resident during the day and a PICU fellow/resident overnight with attending backup), critical care nurse, and a respiratory therapist. |
|
Parshuram 2011 (49) |
Vital signs |
Staff re-training |
NR |
|
McKay 2013 (42) |
Vital signs |
Newly designed ward observation chart, staff training, escalation to senior |
2 tier response: First for bedside nurse to contact child’s primary admitting team to review child. Failure to respond to escalate seniority of MO contacted; MET system continued to be the other formal medical response |
|
Bonafide 2014 (56) |
Vital signs |
MET |
(1) a fellow, attending, or nurse practitioner, (2) a nurse (3) a respiratory therapist |
|
Sefton 2014 (50) |
Vital signs, biochemistry, unresolved pain staff concerns |
Primary/on-call medical/surgical team with a target response |
Existing medical/surgical teams and on call team, ICU consultant as needed |
|
Douglas 2016 (44) |
Vitals, lethargy or confusion, staff or parental concern |
RRT |
PICU Registered Nurse, Respiratory therapist, PICU resident or Nursing Practitioner |
|
Agulnik 2017 (53) |
Vitals, neurological deterioration, cardiac dysrhythmia |
Staff training + modified escalation |
Floor oncologist and PICU physician (same as prior to PEWS implementation) |
|
Kroeger 2018 (48) |
Vital signs, neurological deterioration |
Nursing PEWS - PEWS score is recorded by the ward nursing staff on arrival to the acute care floor |
N/A - used front line staff |
|
Parshuram 2018 (41) |
Vital signs |
Escalation for immediate review |
(If available) Part of existing system in each hospital |
|
Agulnik 2023 (53) |
Vital signs |
Staff training + modified escalation |
Floor oncologist and PICU physician (same as prior to PEWS implementation) |
The results of the quality assessment
The included trial by Parshuram et al. (2018) (41) was a low-risk trial because it was performed with proper randomization and proper blindness. At the same time, the mean score for observational articles was 10,4 out of 14, according to NHLB (tables 3,4).
|
Table 3. Quality assessment for the included studies |
||||||||
|
|
Agulnik 2023 (54) |
Agulnik 2017 (53) |
Anwar-ul-Haque 2010 (55) |
Brilli 2007 (43) |
Sharek 2007 (51) |
Hunt 2008 (49) |
Tibballs 2009 (41) |
Hanson 2010 (45) |
|
1.Was the research question or objective in this paper clearly stated? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
2.Was the study population clearly specified and defined? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
3.Was the participation rate of eligible persons at least 50 %? |
1 |
1 |
1 |
1 |
0 |
1 |
1 |
1 |
|
4.Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? |
0 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
5. Was a sample size justification, power description, or variance and effect estimates |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
6.For the analyses in this paper, were the exposure (s) of interest measured prior to the outcome(s) being measured? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
10. Was the exposure(s) assessed more than once over time? |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
|
11. Were the outcome measures (dependent variables) learly defined, valid, reliable, and implemented consistently across all study participants? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
12. Were the outcome assessors blinded to the exposure status of participants? |
* |
* |
* |
* |
* |
* |
* |
* |
|
13. Was loss to follow-up after baseline 20 % or less? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? |
1 |
0 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Total score (out of 14) |
10/14 |
10/14 |
12/14 |
11/14 |
10/14 |
11/14 |
11/14 |
11/14 |
|
Key: 0 = No, 1 = Yes, N/A = Not applicable, * = Not reported. |
||||||||
|
Table 4. Quality assessment for the included studies |
||||||||
|
|
Kotsakis 2011 (47) |
Parshuram 2011 (49) |
McKay 2013 (42) |
Bonafide 2014 (56) |
Sefton 2014 (50) |
Douglas 2016 (44) |
Kroeger 2018 (48) |
Parshuram 2018 (41) |
|
1.Was the research question or objective in this paper clearly stated? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
2.Was the study population clearly specified and defined? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
3.Was the participation rate of eligible persons at least 50 %? |
1 |
1 |
* |
1 |
1 |
1 |
1 |
1 |
|
4.Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? |
1 |
0 |
0 |
1 |
1 |
1 |
1 |
1 |
|
5. Was a sample size justification, power description, or variance and effect estimates |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
6.For the analyses in this paper, were the exposure (s) of interest measured prior to the outcome(s) being measured? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? |
1 |
1 |
0 |
1 |
1 |
1 |
1 |
1 |
|
8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
10. Was the exposure(s) assessed more than once over time? |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
11. Were the outcome measures (dependent variables) learly defined, valid, reliable, and implemented consistently across all study participants? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
12. Were the outcome assessors blinded to the exposure status of participants? |
* |
* |
* |
* |
* |
* |
* |
* |
|
13. Was loss to follow-up after baseline 20 % or less? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? |
1 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
|
Total score (out of 14) |
11/14 |
10/14 |
7/14 |
11/14 |
10/14 |
10/14 |
10/14 |
10/14 |
|
Key: 0 = No, 1 = Yes, N/A = Not applicable, * = Not reported. |
||||||||
Analysis of the outcomes
Mortality rate
Eleven studies mentioned the rate of mortality and evaluated it. Our analysis revealed that the mortality rate was substantially lower in the PEWS cohort than in the no PEWS cohort (RR= 0,81 [0,69, 0,95], P= 0,01). The analysis showed some heterogeneity as P= 0,0006 and I2 = 68 %. Figure 2 shows the analysis of mortality outcome.
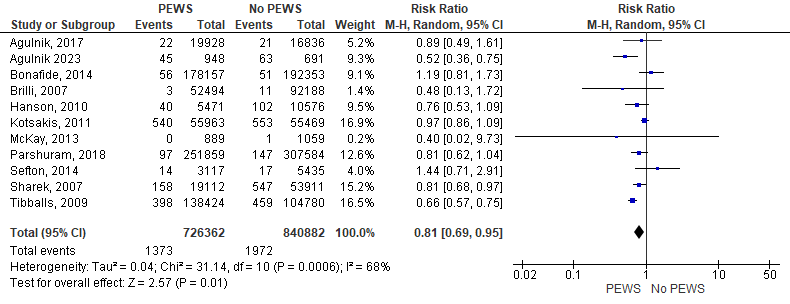
Figure 2. Forest plot showed the analysis of mortality outcome
Cardiopulmonary arrest
This outcome was evaluated by seven articles. The analysis showed that the incidence of cardiopulmonary arrest in no PEWS cohort was significantly higher than that of the PEWS cohort (RR= 0,73 [0,62, 0,85], P= 0,0001). The analysis showed homogeneity as P= 0,37 and I2 = 7 %. Figure 3 shows the analysis of cardiopulmonary arrest outcome.
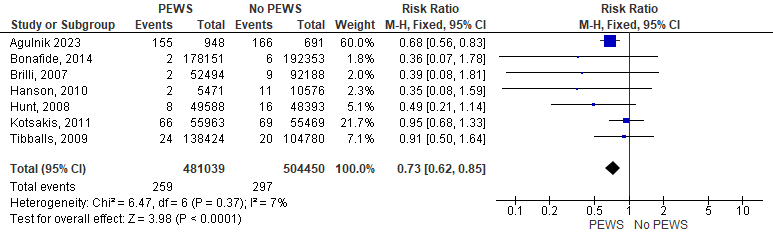
Figure 3. Forest plot showed the analysis of cardiopulmonary arrest outcome
Critical deterioration
Seven studies mentioned the critical deterioration rate of their participants. As a result of the analysis of the data of 975622 patients, we found that the incidence of critical deterioration among patients was higher in the no PEWS cohort than in the PEWS cohort but insignificantly (RR= 0,83 [0,68, 1,02], P= 0,07). The analysis showed some heterogeneity as P= 0,0001 and I2 = 83 %. Figure 4 shows the analysis of critical deterioration outcome.
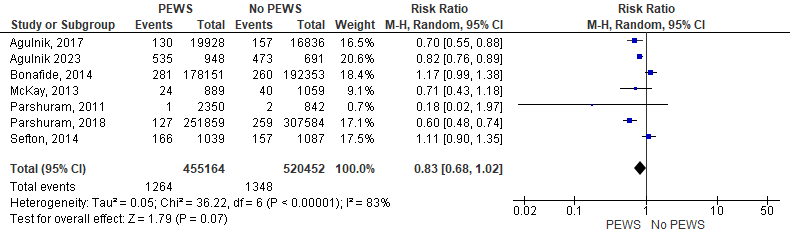
Figure 4. Forest plot showed the analysis of critical deterioration outcome
Unplanned codes
According to the data from four studies that reported this outcome, our analysis revealed that the incidence of unplanned codes was significantly higher in the no PEWS cohort than in the PEWS cohort (RR= 0,66 [0,54, 0,80], P= 0,0001). The analysis showed homogeneity as P= 0,1 and I2 = 52 %. Figure 5 shows the analysis of unplanned codes outcome.
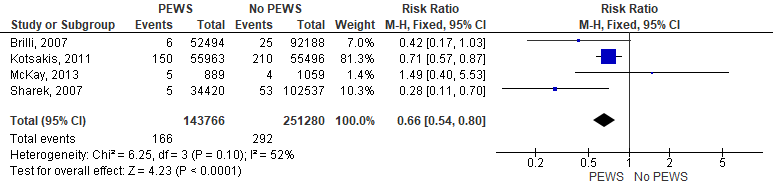
Figure 5. Forest plot showed the analysis of unplanned codes outcome
DISCUSSION
PEWS is a tool used to detect early signs of clinical deterioration in pediatric patients. The development and validation of PEWS tools have been extensively studied in general pediatric populations. Several studies have shown that the use of PEWS can improve the detection of clinical deterioration, reduce the incidence of cardiac arrest, and decrease the length of hospital stay in pediatric patients.(57) This reviews methodically investigated and consolidated research findings on PEWS, which encompasses a holistic system involving detection, response, and implementation elements. However, none of the three review inquiries yielded definitive conclusions regarding the effectiveness and influence of PEWS on clinical practice. In this meta-analysis, PEWS tools were found to be associated with significantly lower incidence of mortality, cardiopulmonary arrest, critical deterioration, and the incidence of unplanned codes.
In a prior comprehensive analysis from 2017, the focus was on evaluating the effectiveness of PEWS in detecting clinical deterioration, as well as the response mechanisms associated with PEWS, and strategies for implementing them. The study revealed positive outcomes in terms of early clinical interventions and potential enhancements in patient safety through collaborative efforts among healthcare professionals. However, it pointed out a lack of standardized measures for robustly comparing results across different studies. The authors also noted the challenge in comparing the performance of PEWS due to variations in components, such as the weighting of physiological parameters, clinical observations, and the thresholds for taking action.(58) It was acknowledged that these differences would persist due to diverse patient populations, as well as disparities in resources and technologies available across different regions and healthcare settings. Hence, the aim of our current pragmatic systematic review was to investigate whether incorporating PEWS, with or without Rapid Response Teams/Medical Emergency Teams (RRT/MET), led to improved clinical outcomes, regardless of the availability of healthcare resources.(58) Several studies have evaluated the effectiveness of PEWS in detecting and managing clinical deterioration in children with oncohematological diseases. A systematic review and meta-analysis by Chong et al.(59) explored the impact of implementing PEWS in hospitals on reducing mortality, cardiopulmonary arrests, unplanned codes, and critical deterioration events in children compared to standard care without PEWS. They also reported similar results. They observed a decrease in both mortality rates and code activations in healthcare systems that adopted PEWS.
They acknowledge that improvements in clinical outcomes are contingent on factors such as the specific healthcare environment, the availability of resources, and the existence of effective response mechanisms. Many studies on PEWS have primarily focused on validating individual tools. A recent systematic review noted that certain pediatric track and trigger tools (PTTT) show good diagnostic accuracy, especially in predicting transfers to the Pediatric Intensive Care Unit (PICU). However, these authors raised methodological concerns that hindered them from making recommendations about the effectiveness of PEWS. Their review encompassed studies involving hospitalized individuals aged 0-18, with outcomes measured in terms of mortality, critical events (such as unplanned transfers to higher levels of care), cardiac and respiratory arrests, immediate medical emergencies, acuity levels at PICU admission, and PICU outcomes. In contrast, our approach was to narrow our search to studies that actually implemented PEWS in their healthcare settings, with a comparative group (“No PEWS”).(60,61,62)
Need for Specific Application and Adaptation of PEWS in Children with Oncohematological Diseases. Children with oncohematological diseases are a unique population that requires specific attention when using PEWS tools. These patients are at increased risk of developing complications due to their underlying disease and the treatments they receive, such as chemotherapy and radiation therapy.
The use of PEWS in children with oncohematological diseases requires specific adaptations to account for the unique physiological changes that occur in these patients. For example, children with leukemia may have a higher heart rate due to anemia or fever, which may not necessarily indicate clinical deterioration. Therefore, modifications to the scoring system may be necessary to avoid false alarms.(63)
The impact of PEWS on patient outcomes in children with oncohematological diseases has been evaluated in several studies. Garza et al.(64) investigated how the implementation of PEWS in hospitals affects the reduction of mortality, cardiopulmonary arrests, unplanned codes, and critical deterioration events in children, compared to standard care without PEWS. This study explored the impact of PEWS on reducing mortality, cardiopulmonary arrests, unplanned codes, and critical deterioration events in children compared to standard care. It also examined how the perceived quality of care during deterioration in children with cancer is influenced by PEWS in different hospital resource settings. The results indicated that healthcare providers caring for children with cancer consider PEWS valuable in improving hospital care, despite challenges such as inadequate critical care resources and technology issues that vary by hospital resource level. These findings reinforce the positive impact of PEWS on quality of care and suggest that it should be widely implemented in clinical practice.(65)
Discrepancies in reported outcomes across studies are likely attributable to differences in patient populations. For example, a decrease in both mortality and code rates was observed by Sharek et al.(51) in a specialized children’s hospital managing complex cases. This hospital serves high-risk pediatric patients, leading to higher pre-intervention code rates compared to other centers. Conversely, institutions with a relatively low frequency of pediatric cardiac arrests suggest that the infrequent occurrence of such events may pose challenges in demonstrating a significant reduction. Despite the potential benefits of using PEWS in children with oncohematological diseases, there are some limitations and challenges that need to be addressed. One of the main challenges is the need for specific adaptations to account for the unique physiological changes that occur in these patients.
Another challenge is the limited evidence regarding the effectiveness of PEWS in this population. Most studies have focused on general pediatric populations, and there is a need for more research to evaluate the effectiveness of PEWS in children with oncohematological diseases.
Limitations
The main limitation of this article is that most of the articles involved are observational studies and involved only one clinical trial. In several of these articles, it was not possible to substantiate a connection between the use of PEWS and better results. Additionally, the implementation of PEWS would have involved hospital-wide education of doctors, nurses, and allied health personnel, and it was impossible to assess the contributions of any of these elements to improvements in outcomes.
CONCLUSION
Our results prove that using PEWS was accompanied by a significant decrease in the rate of unplanned codes, cardiopulmonary arrest, and mortality. However, PEWS showed no effect on the incidence of critical deterioration of the children. Our study reveals the numerous advantages of implementing PEWS for patients, healthcare teams, and institutions, as well as how these advantages interact and support one another. Our study also shows that better patient outcomes improve employee motivation and job satisfaction, and better interpersonal connections foster a better work environment, which in turn leads to changes in hospital culture, including a greater focus on patient safety, patient-centered care, and quality improvement.
PEWS plays an important role in the reduction of rates of mortality, unplanned codes, and cardiopulmonary arrest.
The use of PEWS in children with oncohematological diseases has shown promising results in improving the detection and management of clinical deterioration and reducing the length of hospital stay and morbidity.
However, further research is needed to address the limitations and challenges associated with its use in this population.
PEWS is a systematic approach used in pediatric healthcare settings to identify and monitor signs of clinical deterioration in children.
It involves the assessment and monitoring of specific physiological, behavioral, and clinical parameters to detect early warning signs and facilitate timely interventions.
PEWS tools have been developed and validated, and their application in pediatric healthcare settings aims to improve patient outcomes and reduce morbidity and mortality. However, further research and ongoing evaluation are needed to enhance the implementation and effectiveness of PEWS in different clinical contexts.
REFERENCES
1. Robison J, Slamon NB. A More Rapid, Rapid Response. Pediatr Crit Care Med. 2016 Sep;17(9):871–5. doi:10.1097/PCC.0000000000000855
2. Kurakbayev Y, Turdaliyeva B, Manzhuova L, Omarova K, Abdilova G, Kusainov A, et al. International experience in applying the system of Pediatric Early Warning Signs of critical conditions in oncological children: A literature review. Onkol radiol Kaz [Internet]. 2023;68(2):69–75. Available from: http://dx.doi.org/10.52532/2521-6414-2023-2-68-69-75
3. Agarwal D, Alam S, Mazahir R, Singh RR, Maini B. Utility of Pediatric Early Warning Sign Score in Predicting Outcome of PICU Admissions at a Suburban Tertiary Care Hospital. J Pediatr Intensive Care. 2022 Dec; doi: 10.1055/s-0042-1759730
4. Rickey L, Zhang A, Dean N. Use of Evidence-Based Vital Signs in Pediatric Early Warning Score to Predict Clinical Deterioration on Acute Care Units. Clin Pediatr (Phila). 2023 Apr;000992282311662.
5. McKay V, Chen Y, Prewitt K, Malone S, Puerto-Torres M, Acuña-Aguirre C, et al. Connecting Clinical Capacity and Intervention Sustainability in Resource-Variable Pediatric Oncology Centers in Latin America. Glob Implement Res Appl [Internet]. 2023 Nov 14;4(1):102–15. Available from: https://link.springer.com/10.1007/s43477-023-00106-2
6. Ricci S, Sarli WM, Lodi L, Canessa C, Lippi F, Dini D, et al. HLH as an additional warning sign of inborn errors of immunity beyond familial-HLH in children: a systematic review. Front Immunol [Internet]. 2024 Feb 13;15. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1282804/full
7. McKay V, Carothers B, Graetz D, Malone S, Puerto-Torres M, Prewitt K, et al. Sustainability determinants of an intervention to identify clinical deterioration and improve childhood cancer survival in Latin American hospitals: the INSPIRE study protocol. Implement Sci Commun [Internet]. 2024 Nov 17;4(1):141. Available from: https://implementationsciencecomms.biomedcentral.com/articles/10.1186/s43058-023-00519-y
8. Lambert V, Matthews A, MacDonell R, Fitzsimons J. Paediatric early warning systems for detecting and responding to clinical deterioration in children: a systematic review. BMJ Open. 2017 Mar 13;7(3):e014497. doi: 10.1136/bmjopen-2016-014497. PMID: 28289051; PMCID: PMC5353324.
9. Mirochnick E, Graetz DE, Ferrara G, Puerto-Torres M, Gillipelli SR, Elish P, et al. Multilevel impacts of a pediatric early warning system in resource-limited pediatric oncology hospitals. Front Oncol [Internet]. 2022 Oct 12 [cited 2023 Sep 19];12:1018224. Available from: /pmc/articles/PMC9597682/
10. Hafeez MU, Ali MH, Najib N, Ayub MH, Shafi K, Munir M, Butt NH. Ophthalmic Manifestations of Acute Leukemia. Cureus. 2019 Jan 7;11(1):e3837. doi: 10.7759/cureus.3837.
11. Gauthier M. La leucémie lymphoïde chronique. La Rev Médecine Interne. 2022 Jun;43(6):356–64. https://www.msdmanuals.com/fr/professional/h%C3%A9matologie-et-oncologie/leuc%C3%A9mies/leuc%C3%A9mie-lympho%C3%AFde-chronique-llc
12. Tebbi CK. Etiology of Acute Leukemia: A Review. Cancers (Basel). 2021 May;13(9):2256. doi: 10.3390/cancers13092256
13. Paul S, Rausch CR, Jain N, Kadia T, Ravandi F, DiNardo CD, et al. Treating Leukemia in the Time of COVID-19. Acta Haematol. 2021;144(2):132–45. doi: 10.1159/000508199.
14. Dong Y, Shi O, Zeng Q, Lu X, Wang W, Li Y, et al. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp Hematol Oncol. 2020 Dec;9(1):14. doi: 10.1186/s40164-020-00170-6.
15. Kotb R, Hart C, Goubran H. Multiple Myeloma. In: Paraproteinemia and Related Disorders. Cham: Springer International Publishing; 2022. p. 159–75. https://link.springer.com/chapter/10.1007/978-3-031-10131-1_11.
16. Jurczyszyn A, Charliński G, Vesole DH. Supportive care in multiple myeloma. Acta Haematol Pol. 2022 Aug;53(4):227–40. doi:10.5603/AHP.A2022.0031
17. Veresniuk N. Reforming the Healthcare System to Sustainable Development Goals in the EU States. Econ Aff. 2023 Feb;68(1s):191–8.doi: 10.46852/0424-2513.1s.2023.22.
18. Wallington-Beddoe CT, Mynott RL. Prognostic and predictive biomarker developments in multiple myeloma. J Hematol Oncol. 2021 Dec;14(1):151. doi: 10.1186/s13045-021-01162-7.
19. Parekh I, Virani Z, Rajput P, Vora H, Tapiawala S, Shah B. An unusual presentation of multiple myeloma. Indian J Nephrol. 2022;32(1):79. doi: 10.4103/ijn.IJN_70_21
20. Maltsev DV. A case of persistent recurrent herpes zoster ophthalmicus in a patient with primary mannose binding lectin deficiency. Oftalmol Zh. 2021 Dec;95(6):64–8. doi: 10.31288/OFTALMOLZH202166468.
21. Wang H, Balakrishna JP, Pittaluga S, Jaffe ES. Diagnosis of Hodgkin lymphoma in the modern era. Br J Haematol. 2019 Jan;184(1):45–59. doi: 10.1111/bjh.15614.
22. Lin AY, Schnitter JM, Gordon LI. Immune Checkpoint Blockade for the Treatment of Hodgkin Lymphoma. ImmunoTargets Ther. 2022 Feb;Volume 11:1–10. doi: 10.2147/ITT.S284988.
23. Huang J, Pang WS, Lok V, Zhang L, Lucero-Prisno DE, Xu W, et al. Incidence, mortality, risk factors, and trends for Hodgkin lymphoma: a global data analysis. J Hematol Oncol. 2022 Dec;15(1):57. doi: 10.1186/s13045-022-01281-9.
24. Spînu Ștefan, Cismaru G, Boarescu PM, Istratoaie S, Negru AG, Lazea C, et al. ECG Markers of Cardiovascular Toxicity in Adult and Pediatric Cancer Treatment. Farcaş AD, editor. Dis Markers. 2021 Jan;2021:1–10. doi:10.1155/2021/6653971.
25. Sepbayeva A. Tactics of managing children-patients with post-coagulopathy cardiac surgery interventions. Futur Med. 2022 Dec;34–43. doi: 10.57125/fem.2022.12.30.04.
26. Geel JA, Stevenson BT, Jennings RB, Krook LE, Winnan SJ, Katz BT, et al. Enough is not enough: Medical students’ knowledge of early warning signs of childhood cancer. South African Med J. 2017 Jun;107(7):585. doi: 10.7196/SAMJ.2017.v107i7.12211.
27. Workman GM, Ribeiro RC, Rai SN, Pedrosa A, Workman DE, Pedrosa F. Pediatric cancer knowledge: Assessment of knowledge of warning signs and symptoms for pediatric cancer among Brazilian community health workers. J Cancer Educ. 2007 Sep;22(3):181–5. doi: 10.1007/BF03174334.
28. Suieubekov, B., & Yeshmanova A. New research and changing paradigms of coagulopathy in children after cardiac surgery: A narrative review. Futur Med. 2022 Mar;27–34. doi: 10.57125/fem.2022.03.25.03.
29. Kamel IS. The role of robotics and automation in surgery: critical review of current and emerging technologies. Futur Med. 2023 Mar;23–35. doi: 10.57125/fem.2023.03.30.03.
30. Ntacyabukura B. Childhood Cancer Early Detection Training Program for Primary Healthcare Providers. J Glob Oncol. 2018 Oct;4(Supplement 2):135s-135s. doi: 10.1200/jgo.18.12500.
31. D’alessandro PR, Hamilton J, Khatchadourian K, Lunaczek-Motyka E, Schultz KR, Metzger D, et al. Precocious puberty: A red flag for malignancy in childhood. B C Med J. 2021; https://bcmj.org/articles/precocious-puberty-red-flag-malignancy-childhood.
32. Couitchéré L, Coze C, Atiméré YN, Ouattara J, N’doumy M, Akoun C, et al. Impact d’un programme de diagnostic précoce des cancers de l’enfant à Abidjan ? Bull Cancer. 2021 Mar;108(3):242–9. doi: 10.1016/j.bulcan.2020.11.013.
33. Krishnan Y, Sainulabdin G, Uma VS, Sreedharan PS, Warrier N. Clinical audit of a Paediatric Emergency Warning Score (PEWS) in the paediatric oncology unit of a newly established tertiary cancer institute. Pediatr Hematol Oncol J. 2020 Sep;5(3):69–74. doi: 10.1016/j.phoj.2020.06.006.
34. Lewitan L, Burdach S. Warnsignale für Krebserkrankungen im Kindesalter. Monatsschrift Kinderheilkd. 2021 Jan;169(1):13–9.doi: 10.1007/s00112-020-01081-w.
35. Alimukhamedov U. Acute renal failure in newborns in the practice of a pediatrician of the future. Futur Med. 2022 Dec;17–24. doi: 10.57125/fem.2022.12.30.02.
36. Abstracts from the 52th Congress of the International Society of Paediatric Oncology (SIOP) Virtual Congress, October 14-17, 2020. Pediatr Blood Cancer. 2020 Dec;67 Suppl 4:e28742. doi: 10.1002/pbc.28742. PMID: 33049088.
37. Araz NC, Guler E. Delays in Diagnosis of Childhood Cancer in Southeastern Turkey and the Associated Factors. Pediatr Hematol Oncol. 2015 Feb;32(2):153–63. doi: 10.3109/08880018.2013.874511.
38. Smokova L, Zhylin M, Mendelo V, Kyrylishyna M, Danilova O. Socio-Psychological Factors in the Development of Emotional Intelligence of Drug Addicts. Int J Stat Med Res. 2023 Apr;12:33–42. doi: 10.6000/1929-6029.2023.12.05.
39. Testi AM, Moleti ML, Angi A, Bianchi S, Barberi W, Capria S. Pediatric Autologous Hematopoietic Stem Cell Transplantation: Safety, Efficacy, and Patient Outcomes. Literature Review. Pediatr Heal Med Ther. 2023 May;Volume 14:197–215. doi: 10.2147/phmt.s366636.
40. Atallah FC, Caruso P, Nassar Junior AP, Torelly AP, Amendola CP, Salluh JIF, et al. High-value care for critically ill oncohematological patients: what do we know thus far? Crit Care Sci. 2023;35(1). doi: 10.5935/2965-2774.20230405-en.
41. Parshuram CS, Dryden-Palmer K, Farrell C, Gottesman R, Gray M, Hutchison JS, et al. Effect of a Pediatric Early Warning System on All-Cause Mortality in Hospitalized Pediatric Patients. JAMA. 2018 Mar;319(10):1002. doi:10.1001/jama.2018.0948.
42. McKay H, Mitchell IA, Sinn K, Mugridge H, Lafferty T, Van Leuvan C, et al. Effect of a multifaceted intervention on documentation of vital signs and staff communication regarding deteriorating paediatric patients. J Paediatr Child Health. 2013 Jan;49(1):48–56. doi: 10.1111/jpc.12019.
43. Brilli RJ, Gibson R, Luria JW, Wheeler TA, Shaw J, Linam M, et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit*. Pediatr Crit Care Med. 2007 May;8(3):236–46. doi: 10.1097/01.PCC.0000262947.72442.EA.
44. Douglas K, Collado JC, Keller S. Implementation of a Pediatric Early Warning Scoring System at an Academic Medical Center. Crit Care Nurs Q. 2016 Oct;39(4):363–70. doi: 10.1097/CNQ.0000000000000130.
45. Hanson CC, Randolph GD, Erickson JA, Mayer CM, Bruckel JT, Harris BD, et al. A reduction in cardiac arrests and duration of clinical instability after implementation of a paediatric rapid response system. Postgrad Med J. 2010 May;86(1015):314–8. doi 10.1136/qshc.2007.026054.
46. Hunt EA, Zimmer KP, Rinke ML, Shilkofski NA, Matlin C, Garger C, et al. Transition From a Traditional Code Team to a Medical Emergency Team and Categorization of Cardiopulmonary Arrests in a Children’s Center. Arch Pediatr Adolesc Med. 2008 Feb;162(2):117. doi: 10.1001/archpediatrics.2007.33.
47. Kotsakis A, Lobos AT, Parshuram C, Gilleland J, Gaiteiro R, Mohseni-Bod H, et al. Implementation of a Multicenter Rapid Response System in Pediatric Academic Hospitals Is Effective. Pediatrics. 2011 Jul;128(1):72–8. doi: 10.1542/peds.2010-0756.
48. Kroeger AR, Morrison J, Smith AH. Predicting unplanned readmissions to a pediatric cardiac intensive care unit using predischarge Pediatric Early Warning Scores. Congenit Heart Dis. 2018 Jan;13(1):98–104. doi: 10.1111/chd.12525.
49. Parshuram CS, Bayliss A, Reimer J, Middaugh K, Blanchard N. Implementing the Bedside Paediatric Early Warning System in a community hospital: A prospective observational study. Paediatr Child Health. 2011 Mar;16(3):e18–22. doi: 10.1093/pch/16.3.e18.
50. Sefton G, McGrath C, Tume L, Lane S, Lisboa PJG, Carrol ED. What impact did a Paediatric Early Warning system have on emergency admissions to the paediatric intensive care unit? An observational cohort study. Intensive Crit Care Nurs. 2015 Apr;31(2):91–9. doi: 10.1016/j.iccn.2014.01.001.
51. Sharek PJ, Parast LM, Leong K, Coombs J, Earnest K, Sullivan J, et al. Effect of a Rapid Response Team on Hospital-wide Mortality and Code Rates Outside the ICU in a Children’s Hospital. JAMA. 2007 Nov;298(19):2267. doi: 10.1001/jama.298.19.2267.
52. Tibballs J, Kinney S. Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team*. Pediatr Crit Care Med. 2009 May;10(3):306–12. doi: 10.1097/PCC.0b013e318198b02c.
53. Agulnik A, Mora Robles LN, Forbes PW, Soberanis Vasquez DJ, Mack R, Antillon‐Klussmann F, et al. Improved outcomes after successful implementation of a pediatric early warning system (PEWS) in a resource‐limited pediatric oncology hospital. Cancer. 2017 Aug;123(15):2965–74. doi: 10.1002/cncr.30664.
54. Agulnik A, Muniz-Talavera H, Pham LTD, Chen Y, Carrillo AK, Cárdenas-Aguirre A, et al. Effect of paediatric early warning systems (PEWS) implementation on clinical deterioration event mortality among children with cancer in resource-limited hospitals in Latin America: a prospective, multicentre cohort study. Lancet Oncol. 2023 Sep;24(9):978–88. doi: 10.1016/S1470-2045(23)00285-1.
55. Anwar-ul-Haque, Saleem AF, Zaidi S, Haider SR. Experience of pediatric rapid response team in a tertiary care hospital in Pakistan. Indian J Pediatr. 2010 Mar;77(3):273–6. doi: 10.1007/s12098-010-0032-2.
56. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of Rapid Response System Implementation on Critical Deterioration Events in Children. JAMA Pediatr. 2014 Jan;168(1):25. doi: 10.1001/jamapediatrics.2013.3266.
57. Sánchez-Ramón S, Bermúdez A, González-Granado LI, Rodríguez-Gallego C, Sastre A, Soler-Palacín P, et al. Primary and Secondary Immunodeficiency Diseases in Oncohaema/tology: Warning Signs, Diagnosis, and Management. Front Immunol [Internet]. 2019 [cited 2023 Sep 19];10(MAR). Available from: https://pubmed.ncbi.nlm.nih.gov/30984175/
58. Lambert V, Matthews A, MacDonell R, Fitzsimons J. Paediatric early warning systems for detecting and responding to clinical deterioration in children: a systematic review. BMJ Open [Internet]. 2017 Mar 1 [cited 2023 Oct 25];7(3). Available from: https://pubmed.ncbi.nlm.nih.gov/28289051/
59. Chong SL, Goh MSL, Ong GYK, Acworth J, Sultana R, Yao SHW, et al. Do paediatric early warning systems reduce mortality and critical deterioration events among children? A systematic review and meta-analysis. Resusc Plus [Internet]. 2022 Sep 1 [cited 2023 Sep 19];11:100262. Available from: /pmc/articles/PMC9253845/
60. Trubey R, Huang C, Lugg-Widger F V., Hood K, Allen D, Edwards D, et al. Validity and effectiveness of paediatric early warning systems and track and trigger tools for identifying and reducing clinical deterioration in hospitalised children: a systematic review. BMJ Open [Internet]. 2019 May 1 [cited 2023 Oct 25];9(5):e022105. Available from: https://bmjopen.bmj.com/content/9/5/e022105
61. Pérez Salazar NN, Tabares Rosero LG. Variation of hematological and biochemical profile in dialyzed patients before and after this treatment. Salud, Ciencia y Tecnología. 2024;4:762.
62. Montes Hijar EP, Cuyubamba Pérez EE, Manrique Meza JH, Hinojo Veliz DI. Regulatory Compliance and Managerial Control in the Hemotherapy and Blood Bank Program of EsSalud Huancayo. Salud, Ciencia y Tecnología. 2024;4:1002
63. Duncan H, Hutchison J, Parshuram CS. The pediatric early warning system score: A severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006 Sep 1;21(3):271–8. doi: 10.1016/j.jcrc.2006.06.007.
64. Garza M, Graetz DE, Kaye EC, Ferrara G, Rodriguez M, Soberanis Vásquez DJ, et al. Impact of PEWS on Perceived Quality of Care During Deterioration in Children With Cancer Hospitalized in Different Resource-Settings. Front Oncol [Internet]. 2021 Jun 23 [cited 2023 Sep 19];11:660051. Available from: /pmc/articles/PMC8260684/
65. Abdala F, Khalifa M, Okby O, Fathala A. Effect of an Evidence Based Nursing Intervention on the Early Detection of Pediatric Warning Signs. Menoufia Nurs J. 2018;3(1):137–45. doi: 10.21608/menj.2018.151631.
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Yedil Kurakbayev, Kuanysh Umbetov, Yergali Sarsekbayev.
Data curation: Botagoz Turdaliyeva, Lyazat Manzhuova.
Formal analysis: Yergali Sarsekbayev, Kuanysh Umbetov.
Research: Yedil Kurakbayev, Lyazat Manzhuova.
Methodology: Yedil Kurakbayev, Botagoz Turdaliyeva.
Project management: Yedil Kurakbayev.
Software: Kuanysh Umbetov.
Supervision: Yedil Kurakbayev, Yergali Sarsekbayev.
Validation: Botagoz Turdaliyeva, Lyazat Manzhuova.
Display: Abay Kussainov, Gulnara Abdilova.
Drafting - original draft: Yedil Kurakbayev, Botagoz Turdaliyeva, Kuanysh Umbetov.
Writing - proofreading and editing: Yedil Kurakbayev, Lyazat Manzhuova, Yergali Sarsekbayev.