doi: 10.56294/saludcyt20241285
ORIGINAL
AI in the Development of Vaccines for Emerging and Re-Emerging Diseases
IA en el Desarrollo de Vacunas para Enfermedades Emergentes y Reemergentes
Rita
Elizabeth Velastegui-Hernandez1
![]() , Veronica Gabriela Salinas-Velastegui1
, Veronica Gabriela Salinas-Velastegui1
![]() , Diana Catalina Velastegui-Hernández1
, Diana Catalina Velastegui-Hernández1
![]() , Estefania Araceli Reyes-Rosero1
, Estefania Araceli Reyes-Rosero1
![]() , Andrea Carolina Cevallos-Teneda1
, Andrea Carolina Cevallos-Teneda1
![]() , Andrea Alexandra Tufiño-Aguilar1
, Andrea Alexandra Tufiño-Aguilar1
![]() , Fabricio Alejandro Vasquez de la Bandera1
, Fabricio Alejandro Vasquez de la Bandera1
![]() , Gabriela Sandoval1
, Gabriela Sandoval1
![]() , Luis Felipe Contreras-Vásquez2
, Luis Felipe Contreras-Vásquez2
![]() *,
Luis Fabián Salazar-Garcés1,3
*,
Luis Fabián Salazar-Garcés1,3
![]() *
*
1Technical University of Ambato, Faculty of Health Sciences. Ambato, Ecuador.
2Research and Development Directorate, Faculty of Civil and Mechanical Engineering. Ambato, Ecuador.
3Federal University of Bahia, Institute of Health Sciences. Salvador, Bahia, Brazil.
Cite as: Velastegui-Hernandez RE, Salinas-Velastegui VG, Velastegui-Hernández DC, Reyes-Rosero EA, Cevallos-Teneda AC, Tufiño-Aguilar AA, et al. AI in the Development of Vaccines for Emerging and Re-Emerging Diseases. Salud, Ciencia y Tecnología. 2024; 4:1285. https://doi.org/10.56294/saludcyt20241285
Submitted: 03-02-2024 Revised: 12-07-2024 Accepted: 02-12-2024 Published: 03-12-2024
Editor: Prof.
Dr. William Castillo-González ![]()
Corresponding Author: Luis Fabián Salazar-Garcés *
ABSTRACT
Introduction: the integration of artificial intelligence (AI) into vaccine development has revolutionized traditional methodologies, significantly enhancing the speed, precision, and scalability of immunological research. Emerging and re-emerging infectious diseases, driven by zoonotic spillovers, antimicrobial resistance, and global environmental changes, pose substantial challenges. Addressing these requires innovative approaches, with AI playing a pivotal role in advancing immunological solutions.
Development: AI applications in vaccinology include antigen detection, adjuvant optimization, and immune response simulation. Deep learning algorithms streamline the identification of immunogenic targets and conserved antigens, enabling vaccine development for highly mutable pathogens such as SARS-CoV-2, HIV, and influenza. Case studies demonstrate AI’s transformative impact, including its role in the rapid creation of mRNA vaccines for COVID-19, identification of promising antigens for malaria, and enhanced efficacy of influenza vaccines through predictive modeling. However, challenges such as unequal access to technology, biases in data models, and ethical concerns regarding genomic data privacy persist. Recommendations to address these barriers include increasing data diversity, strengthening ethical frameworks, and investing in global infrastructure to democratize AI-driven innovations.
Conclusions: AI’s ability to reduce time and cost, improve vaccine precision, and enable personalized immunization strategies positions it as a cornerstone of modern vaccinology. With continued advancements and equitable implementation, AI holds the potential to reshape vaccine development, improve pandemic preparedness, and address longstanding public health disparities globally.
Keywords: Artificial Intelligence; Vaccine Development; Emerging Diseases; Immunology; Genomic Data Ethics.
RESUMEN
Introducción: la integración de la inteligencia artificial (IA) en el desarrollo de vacunas ha revolucionado las metodologías tradicionales, mejorando significativamente la velocidad, precisión y escalabilidad de la investigación inmunológica. Las enfermedades infecciosas emergentes y reemergentes, impulsadas por la transmisión zoonótica, la resistencia a los antimicrobianos y los cambios ambientales globales, representan desafíos sustanciales. Abordar estos problemas requiere enfoques innovadores, con la IA desempeñando un papel fundamental en el avance de soluciones inmunológicas.
Desarrollo: las aplicaciones de la IA en la vacunología incluyen la detección de antígenos, la optimización de adyuvantes y la simulación de respuestas inmunológicas. Los algoritmos de aprendizaje profundo agilizan la identificación de objetivos inmunogénicos y antígenos conservados, lo que permite el desarrollo de vacunas para patógenos altamente mutables como el SARS-CoV-2, el VIH y la influenza. Los estudios de caso demuestran el impacto transformador de la IA, incluido su papel en la rápida creación de vacunas de ARNm para la COVID-19, la identificación de antígenos prometedores para la malaria y la mejora de la eficacia de las vacunas contra la influenza mediante modelos predictivos. Sin embargo, persisten desafíos como el acceso desigual a la tecnología, los sesgos en los modelos de datos y las preocupaciones éticas relacionadas con la privacidad de los datos genómicos. Entre las recomendaciones para abordar estas barreras se incluyen el aumento de la diversidad de datos, el fortalecimiento de los marcos éticos y la inversión en infraestructura global para democratizar las innovaciones impulsadas por la IA.
Conclusiones: la capacidad de la IA para reducir el tiempo y los costos, mejorar la precisión de las vacunas y permitir estrategias de inmunización personalizadas la posiciona como un pilar fundamental de la vacunología moderna. Con avances continuos e implementación equitativa, la IA tiene el potencial de transformar el desarrollo de vacunas, mejorar la preparación ante pandemias y abordar las disparidades de salud pública que persisten a nivel mundial.
Palabras clave: Inteligencia Artificial; Desarrollo de Vacunas; Enfermedades Emergentes; Inmunología; Ética de Datos Genómicos.
INTRODUCTION
The rapid advancement of high-throughput sequencing technologies has significantly The emergence and re-emergence of infectious diseases pose a persistent and growing threat to global health. Over the past few decades, outbreaks of novel pathogens such as SARS-CoV-2, Zika virus, and Ebola virus, as well as the resurgence of diseases like tuberculosis and dengue fever, have underscored the critical need for innovative and efficient approaches to vaccine development.(1,2) Traditional vaccine development methods, while successful in many instances, often require years of research and extensive resources, limiting their capacity to respond promptly to rapidly evolving pathogens.(3,4) This limitation has prompted the integration of advanced technologies, with artificial intelligence (AI) emerging as a transformative tool in the field of vaccinology.(4)
Artificial intelligence, defined as the simulation of human intelligence processes by computer systems, has revolutionized numerous sectors, including healthcare, pharmaceuticals, and biotechnology.(5,6) In the context of vaccine development, AI offers unparalleled capabilities in processing complex biological data, predicting antigenic targets, and optimizing vaccine candidates with unprecedented speed and precision.(7,8) These capabilities are particularly vital in addressing the challenges posed by emerging and re-emerging diseases, which often involve pathogens with high mutation rates, geographic variability, and limited pre-existing research.
This review aims to provide a comprehensive examination of the role of AI in vaccine development for emerging and re-emerging diseases. It will explore the applications of AI in antigen identification, adjuvant optimization, and immune response simulation, highlighting its contributions to accelerating the development process and improving vaccine efficacy.(9,10) Furthermore, the review will discuss the use of AI in predicting pathogen evolution, enabling the design of vaccines that anticipate future mutations.
Despite its transformative potential, the application of AI in vaccine development is not without challenges. Issues such as data privacy, algorithmic bias, and access to technology remain critical barriers that need to be addressed to ensure equitable and effective implementation.(11) These limitations, along with the ethical and regulatory considerations, will also be examined in this review to provide a balanced perspective on the integration of AI in vaccinology.
This article is structured into several key sections. First, it will present the current landscape of emerging and re-emerging diseases and the challenges they pose to traditional vaccine development. Next, the applications of AI in various stages of vaccine development will be detailed, followed by an analysis of the benefits and challenges associated with its use. Case studies of successful AI-driven vaccine initiatives will be highlighted to illustrate its real-world impact. Finally, the review will outline future directions and offer recommendations for enhancing the role of AI in addressing global health challenges.
By synthesizing recent advancements and identifying both opportunities and challenges, this review aims to contribute to the growing body of knowledge on AI's role in addressing one of the most pressing issues in global health: the timely and effective development of vaccines for emerging and re-emerging infectious diseases (figure 1).
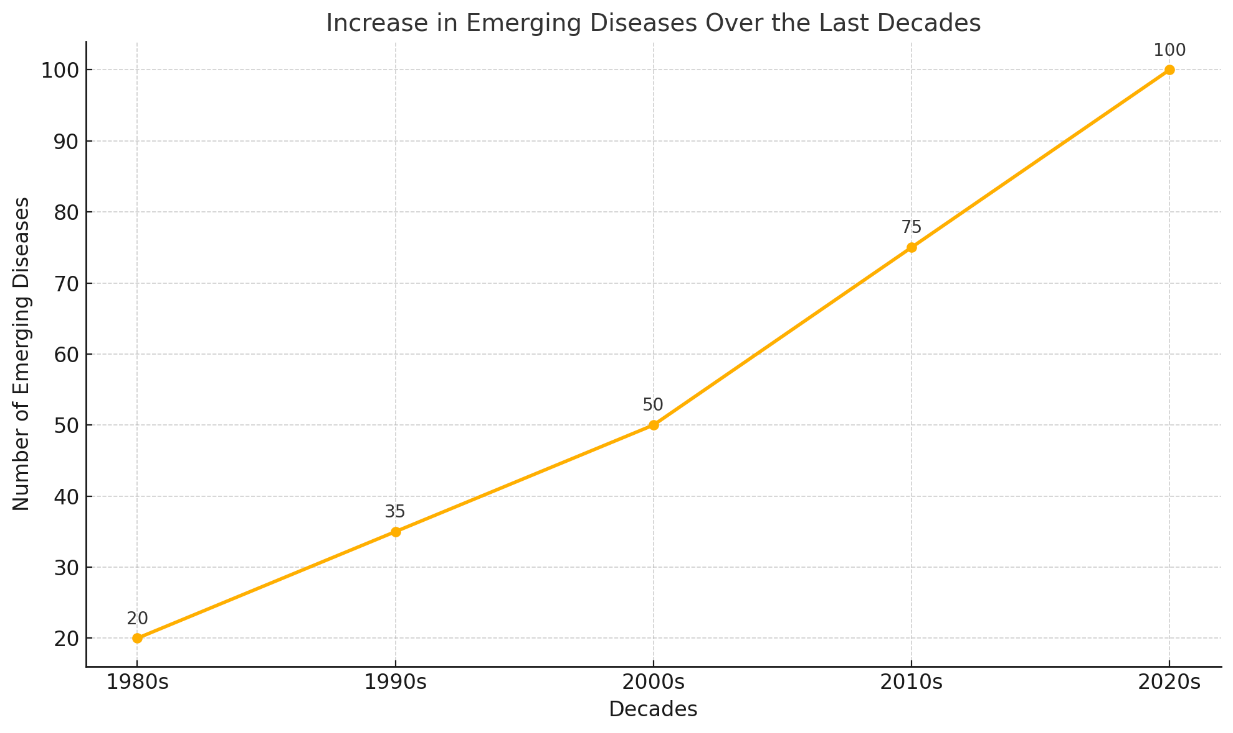
Figure 1. Increase in emerging diseases over the last decades
METHOD
The methodological approach employed in this review involved a comprehensive and systematic analysis of peer-reviewed literature, case studies, and relevant technical reports that examine the role of artificial intelligence (AI) in vaccine development. The selection criteria focused on publications that address key areas such as antigen identification, adjuvant optimization, immune response simulation, and predictive modeling of pathogen evolution. Articles were sourced primarily from indexed databases including PubMed, Scopus, and IEEE Xplore, ensuring a broad representation of multidisciplinary research.
To identify relevant studies, we conducted a keyword-based search using terms such as "artificial intelligence," "vaccine development," "emerging diseases," and "immunoinformatics." The search strategy included Boolean operators to capture intersections between AI applications and vaccine-related innovations. Studies published within the last five years were prioritized to ensure the inclusion of the latest advancements, though seminal works predating this period were also considered where foundational context was required. The inclusion criteria emphasized studies providing empirical evidence, computational models, or real-world applications of AI in the field of vaccinology.
For the analysis, all selected studies were evaluated for their scientific rigor, relevance to the review's objectives, and the novelty of their findings. Data extracted from the studies were synthesized to identify recurring themes, emerging trends, and significant case studies illustrating the transformative potential of AI. Particular emphasis was placed on highlighting both the benefits and limitations of AI-driven approaches, ensuring a balanced perspective that considers technical, ethical, and practical implications.
This methodological framework underpins the organization and structure of the review, ensuring that it provides a comprehensive and nuanced understanding of the intersection between AI and vaccine development for emerging and re-emerging diseases.
Emerging and re-emerging diseases: current context
Definition and Characteristics
Emerging infectious diseases (EIDs) are defined as diseases caused by previously unknown or uncharacterized pathogens, or those that are experiencing significant increases in incidence or geographic range.(12,13) These diseases often arise due to zoonotic spillovers, where pathogens from animals infect humans, facilitated by environmental changes, urbanization, and global travel.(14) Examples include zoonotic diseases like Ebola and Zika virus, which emerged as major threats in recent decades.(15)
Re-emerging infectious diseases (RIDs), on the other hand, refer to diseases that were once under control but have reappeared in populations, often due to declining vaccination rates, antimicrobial resistance, or ecological changes.(16) Diseases such as tuberculosis (TB), dengue fever, and cholera illustrate this category. Unlike EIDs, RIDs are often exacerbated by social determinants of health, including poverty and access to medical infrastructure.(13,17) Together, EIDs and RIDs challenge public health systems globally, creating an urgent need for efficient and scalable vaccine development methods.
Differences Between Emerging and Re-Emerging Diseases
While both categories share the characteristic of posing renewed or novel threats to public health, their epidemiological and etiological profiles differ significantly.(18,19) Emerging diseases often result from novel pathogens, requiring entirely new strategies for diagnosis, treatment, and prevention.(20,21) The COVID-19 pandemic, caused by SARS-CoV-2, is a quintessential example of an EID that necessitated the rapid development of diagnostics, therapeutics, and vaccines.(22)
Conversely, re-emerging diseases often reappear due to gaps in public health measures or the emergence of drug-resistant strains.(5) For instance, the resurgence of tuberculosis has been linked to multidrug-resistant (MDR) and extensively drug-resistant (XDR) Mycobacterium tuberculosis strains.(23,24) Addressing RIDs often involves revitalizing existing interventions rather than developing entirely new tools, though the evolution of pathogens can blur these distinctions.(25,26) Understanding these differences is crucial to tailoring vaccine development strategies appropriately.
Recent Examples: COVID-19, Tuberculosis, and Avian Influenza
The COVID-19 pandemic represents one of the most significant public health challenges in recent history.(27) Its rapid global spread highlighted the vulnerabilities of existing vaccine development pipelines.(28) Within a year of its discovery, AI-assisted approaches were instrumental in the identification of SARS-CoV-2 antigens, leading to the development of mRNA vaccines such as those from Pfizer-BioNTech and Moderna.(29) The unprecedented speed of these developments showcased the potential of AI to overcome traditional vaccine limitations.
Tuberculosis, a classic example of a re-emerging disease, remains one of the leading causes of death globally despite the availability of the Bacille Calmette-Guérin (BCG) vaccine.(30) The rise of MDR and XDR strains has rendered many traditional treatments ineffective, necessitating the exploration of novel vaccine candidates.(31) AI has been employed in genome-wide association studies to identify antigenic targets for these resistant strains, offering hope for improved vaccines.
Avian influenza viruses, particularly highly pathogenic strains such as H5N1 and H7N9, continue to pose threats due to their potential for zoonotic transmission and pandemic outbreaks.(32) Their genetic variability complicates the design of universal vaccines. AI-based predictive models have been utilized to analyze viral genomes and predict antigenic drift, aiding in the formulation of seasonal and pandemic influenza vaccines.(32) The most relevant findings of this section are summarized in table 1.
|
Table 1. Expanded_Comparison_of_Emerging_and_Re-Emerging_Diseases |
|||||
|
Category |
Definition |
Examples |
Key Drivers |
Challenges |
Strategies for Mitigation |
|
Emerging Diseases |
Diseases caused by previously unknown pathogens, zoonotic spillovers, or those significantly increasing in incidence or geographic range due to factors such as globalization, climate change, and urbanization. |
COVID-19, Zika virus, Ebola virus, Nipah virus, MERS-CoV |
Environmental changes, increased human-animal interactions, and global travel facilitating the spread of novel pathogens. |
High mutation rates, limited prior knowledge of pathogens, lack of existing vaccines or treatments, and the urgent need for rapid development of effective interventions. |
Real-time genomic surveillance, AI-driven pathogen tracking, vaccine platform technologies, and international collaboration for rapid response. |
|
Re-Emerging Diseases |
Diseases that were previously under control or had declining incidence but have reappeared due to antimicrobial resistance, gaps in vaccination programs, socioeconomic factors, or environmental changes. |
Tuberculosis, Dengue fever, Cholera, Measles, Polio |
Reduced vaccination coverage, drug resistance, breakdowns in healthcare infrastructure, and changes in environmental conditions. |
Emergence of resistant strains, public health policy failures, reintroduction in naive populations, and the complexity of controlling outbreaks. |
Strengthening public health infrastructure, enhancing vaccine coverage, monitoring resistance patterns, and increasing global health investments. |
Challenges in vaccine development for emerging and re-emerging diseases
Genetic Variability of Pathogens
One of the primary challenges in developing vaccines for both EIDs and RIDs is the high genetic variability of pathogens. RNA viruses, such as SARS-CoV-2 and influenza, mutate rapidly, complicating the identification of stable antigens.(33) For example, SARS-CoV-2 variants such as Delta and Omicron demonstrated immune escape capabilities, reducing vaccine efficacy and necessitating the development of updated formulations.(34)
Similarly, tuberculosis’s evolution into MDR and XDR strains underscores the importance of targeting conserved antigens.(35) AI-driven computational approaches have been employed to predict mutation-prone regions in pathogen genomes, enabling the design of vaccines that are more likely to remain effective despite genetic shifts.(36)
Limitations of Traditional Methods
Traditional vaccine development methods, which rely heavily on trial-and-error approaches in identifying antigens and adjuvants, are often too slow to meet the demands of modern public health crises.(37,38) The conventional pipeline for vaccine development can take over a decade, involving extensive preclinical and clinical testing phases.(39) This lag time was evident during the early stages of the HIV/AIDS epidemic, where vaccine candidates failed to provide sufficient protection despite decades of research.(40)
AI has the potential to transform this paradigm by streamlining antigen discovery, optimizing adjuvants, and simulating immune responses in silico.(10) For example, machine learning algorithms can analyze massive datasets from pathogen genomes to identify conserved regions with high immunogenic potential, significantly reducing the time required for vaccine candidate selection.(29)
Need for Rapid and Precise Solutions
Emerging infectious diseases demand rapid responses due to their potential for exponential spread, while re-emerging diseases require precise interventions to address existing public health challenges.(41,42) The rapid deployment of mRNA vaccines for COVID-19 demonstrated the power of leveraging advanced technologies for speed and precision.(41) However, many diseases, such as tuberculosis, remain underserved by these innovations due to disparities in resource allocation and technological accessibility.(43)
The integration of AI into vaccine development is particularly valuable in addressing these dual needs. By employing predictive models to simulate vaccine efficacy and adaptive immune responses, researchers can develop and refine vaccine candidates with unprecedented efficiency.(44,45) These capabilities are critical in ensuring that vaccines can be deployed swiftly while maintaining high efficacy rates.
Applications of Artificial Intelligence in Vaccine Design and Development
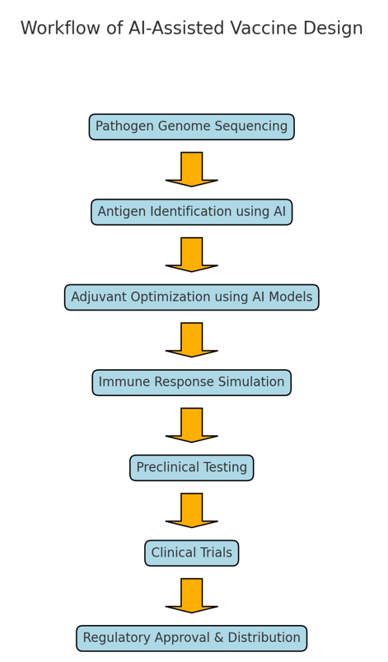
Figure 2. AI-assisted vaccine design workflow
Artificial intelligence (AI) has emerged as a transformative tool in vaccine design and development, enabling unprecedented advancements in antigen detection, adjuvant optimization, and immune response simulation.(10,46,47) By leveraging machine learning algorithms, particularly deep learning, researchers can address the challenges posed by emerging and re-emerging diseases, including the genetic variability of pathogens and the need for rapid vaccine production.(48,49) This section examines three critical applications of AI in vaccine development: antigen detection, adjuvant optimization, and the simulation of immune responses (figure 2).
Antigen Detection Through Artificial Intelligence
The identification of antigens, particularly epitopes that trigger immune responses, is a foundational step in vaccine design.(47) AI, specifically deep learning algorithms, has revolutionized this process by enabling the rapid analysis of vast genomic and proteomic datasets to identify promising antigenic targets.(50)
Deep Learning Algorithms in Epitope Identification
Deep learning, a subset of machine learning, utilizes neural networks to analyze patterns in data.(51) In the context of vaccine development, these algorithms process high-throughput sequencing data to identify epitopes—specific regions of antigens recognized by the immune system.(52,53) Traditional methods for epitope identification often rely on labor-intensive techniques, such as X-ray crystallography or manual curation of pathogen databases.(54) AI simplifies and accelerates this process by automating the identification of conserved and immunogenic regions within a pathogen's genome or proteome.(55)
For example, convolutional neural networks (CNNs) and recurrent neural networks (RNNs) have been employed to predict both linear and conformational epitopes with high accuracy.(56) Tools such as NetMHC and DeepVacPred use machine learning to predict major histocompatibility complex (MHC) binding affinities, a critical factor in determining epitope immunogenicity.(57,58) These approaches significantly reduce the time required to identify candidates for experimental validation, thereby accelerating the vaccine development timeline.
Case Study: SARS-CoV-2 and mRNA Vaccines
The COVID-19 pandemic showcased the potential of AI in antigen discovery. Researchers used AI-driven tools to rapidly analyze the SARS-CoV-2 genome and identify the spike (S) protein as the primary antigenic target for vaccine development.(37,59) AI models predicted key regions within the S protein, such as the receptor-binding domain (RBD), that were most likely to elicit robust immune responses.(60,61) These predictions formed the basis of mRNA vaccines developed by Pfizer-BioNTech and Moderna.
Unlike traditional vaccines, mRNA vaccines require the synthesis of genetic instructions encoding the antigen.(62) AI-assisted antigen prediction reduced the time needed to design these instructions, enabling the rapid development of vaccines that have demonstrated high efficacy against COVID-19.(63,64) This approach has set a new standard for vaccine development against emerging pathogens.
Optimization of Adjuvants
Adjuvants are compounds added to vaccines to enhance the immune response, making them a critical component of vaccine efficacy.(65) Despite their importance, adjuvant discovery has historically been a trial-and-error process.(66) AI has transformed this paradigm by enabling the rational design and optimization of adjuvants using predictive modeling.
Enhancing Immune Response with AI
AI-driven models analyze vast chemical and biological datasets to predict the immunostimulatory potential of novel adjuvants.(67) These models incorporate data on molecular structure, receptor binding, and immunogenic pathways to identify compounds likely to enhance vaccine efficacy.(68) For example, machine learning algorithms have been used to predict Toll-like receptor (TLR) agonists, a class of adjuvants that activate innate immunity.(69)
AI also enables the customization of adjuvants to specific pathogens or populations.(46) For instance, by analyzing population-level genomic data, AI can predict adjuvants that optimize immune responses in individuals with varying MHC genotypes.(70) This approach holds promise for developing vaccines tailored to specific regions or demographics.
Examples of AI-Discovered Adjuvants
Several adjuvants have been identified or optimized using AI. For example, the use of computational models in the development of AS03, an adjuvant used in influenza vaccines, demonstrated the potential of AI to streamline adjuvant discovery.(71) Similarly, AI-driven molecular docking simulations have identified novel adjuvants for vaccines targeting diseases such as malaria and tuberculosis.(10,46) These discoveries underscore the ability of AI to enhance the efficacy of vaccines while reducing the time and cost associated with adjuvant development.
Simulation of immune responses
Once antigens and adjuvants are identified, understanding how they elicit immune responses is essential.(72) Traditionally, immune responses have been studied through in vitro and in vivo experiments, which are time-consuming and resource-intensive.(73,74) AI offers an alternative by simulating immune responses in silico, providing valuable insights into vaccine efficacy and safety.
AI Models for Immune Response Simulation
AI-based simulation models integrate data from immunological studies to predict how a candidate vaccine will interact with the immune system.(10,75) These models use machine learning to simulate complex interactions between antigens, adjuvants, and immune cells, enabling researchers to evaluate vaccine performance before initiating clinical trials.(47,75)
For instance, agent-based models (ABMs) and systems biology approaches have been employed to simulate the dynamics of immune cell activation and antibody production.(76,77) These models provide a detailed understanding of immune pathways, such as the activation of T cells and B cells, and predict the strength and duration of immune responses.(60,78) AI simulations also help identify potential safety concerns, such as adverse immune reactions, reducing the risk of failure in later stages of development.
Benefits for Clinical Trials
The use of AI in immune response simulation offers significant advantages for vaccine development.(10,75) By predicting the immunogenicity of vaccine candidates, AI can prioritize the most promising candidates for experimental validation, thereby reducing the number of candidates requiring costly and time-intensive preclinical testing.(79,80) This approach was particularly valuable during the COVID-19 pandemic, where AI simulations played a key role in evaluating the efficacy of multiple vaccine candidates simultaneously.(63,75,81)
AI models also support adaptive trial designs by analyzing interim data to refine dosing regimens and optimize trial outcomes.(82,83) For example, Bayesian optimization algorithms have been used to adjust vaccine dosing schedules based on real-time data, ensuring that trials are both efficient and scientifically rigorous.(84,85) These capabilities demonstrate the potential of AI to reduce the time and cost associated with clinical trials while improving the likelihood of successful outcomes.
AI’s applications in antigen detection, adjuvant optimization, and immune response simulation represent a paradigm shift in vaccine development.(37,86) These tools have enabled researchers to overcome longstanding challenges, including the variability of pathogens and the limitations of traditional methods, by leveraging data-driven insights.(48,87) As vaccine development continues to evolve, AI will play an increasingly central role in addressing the complexities of emerging and re-emerging diseases, paving the way for more efficient and effective vaccination strategies.
AI in predicting pathogen evolution
The rapid mutation and adaptation of pathogens, particularly RNA viruses, pose significant challenges to vaccine development. Artificial intelligence (AI) has become an indispensable tool in predicting the evolutionary trajectories of pathogens, enabling researchers to anticipate viral mutations and design vaccines that remain effective despite genetic variations.(48,88) This section explores the application of predictive AI models in understanding pathogen evolution and their implications for future vaccine development.
Predictive Models for Mutations
Anticipating the Evolution of Highly Mutable Viruses
RNA viruses, such as influenza and HIV, exhibit high mutation rates due to their error-prone replication mechanisms.(89) This genetic variability allows them to evade host immune responses and reduce the efficacy of vaccines.(90) AI-driven predictive models analyze viral genomic data to identify patterns of mutation and forecast evolutionary changes.(91) By training machine learning algorithms on historical mutation data, researchers can predict how specific regions of a virus's genome are likely to evolve, aiding in the proactive design of vaccines.(92,93)
For example, in the case of influenza, predictive models such as Nextstrain leverage phylogenetic and machine learning approaches to track viral evolution in real-time.(94) These models identify genetic drift and shift patterns, enabling public health authorities to select vaccine strains that are more likely to be effective in the upcoming flu season.(95) Similarly, for HIV, deep learning algorithms have been applied to predict escape mutations in viral epitopes targeted by immune responses, guiding the development of vaccines with broad efficacy.(96,97,98)
Case Study: Predicting Variants of SARS-CoV-2
The emergence of SARS-CoV-2 variants during the COVID-19 pandemic highlighted the importance of predictive modeling in vaccine design. AI models were instrumental in identifying mutations in the virus’s spike (S) protein, which mediates host cell entry and is the primary target of vaccines.(99,100) For instance, machine learning algorithms analyzed genomic sequences from global SARS-CoV-2 databases to predict mutations in the receptor-binding domain (RBD), such as those seen in the Delta and Omicron variants.(99,101)
These predictions allowed researchers to rapidly update vaccine formulations and develop booster doses targeting these variants. The proactive identification of mutations also informed public health measures, such as enhanced genomic surveillance and travel restrictions.(102,103) By enabling real-time tracking and forecasting of variant evolution, AI has proven indispensable in managing the COVID-19 pandemic and preparing for future viral outbreaks.
Impact on the design of future vaccines
Designing Universal Vaccines Based on Evolutionary Patterns
One of the most ambitious applications of predictive AI models is the design of universal vaccines that provide long-lasting protection against a wide range of viral strains.(104,105) By analyzing patterns of evolutionary conservation across diverse viral genomes, AI can identify "conserved regions" that are less prone to mutation.(106,107) These regions serve as ideal targets for vaccine development, as they are shared across multiple strains of a virus and elicit robust immune responses.(108,109)
For example, in influenza vaccine research, AI has identified conserved epitopes in the hemagglutinin (HA) and neuraminidase (NA) proteins, which are essential for viral replication and infectivity.(110) Targeting these epitopes has the potential to create universal flu vaccines that remain effective despite antigenic drift and shift.(111) Similarly, in the context of HIV, AI has been used to identify conserved regions of the virus's envelope protein, informing the design of broadly neutralizing antibody (bNAb)-based vaccines.(111,112)
Current Limitations of Predictive Models
Despite their potential, predictive models face several limitations that impact their application in vaccine development.(113) A major challenge is the availability and quality of data used to train these models. In many cases, genomic datasets lack representation from diverse geographic regions, leading to biases in predictions.(114,115) For example, AI models trained primarily on SARS-CoV-2 sequences from high-income countries may fail to account for mutations arising in underrepresented regions, reducing their global applicability.(116)
Additionally, the dynamic nature of viral evolution introduces uncertainty into predictions. While AI can identify likely mutation hotspots, it cannot fully account for the complex interplay of selective pressures, such as host immunity and antiviral treatments, that drive evolution.(105,117) This uncertainty underscores the need for continuous refinement of models and integration with real-time genomic surveillance systems.
Another limitation is the computational complexity of predictive models. Deep learning algorithms often require significant computational resources, which may not be accessible to all research institutions, particularly in low- and middle-income countries.(118,119) Ensuring equitable access to AI tools and resources is critical for maximizing their impact on global vaccine development efforts.(120)
AI’s capacity to predict pathogen evolution represents a paradigm shift in vaccine development, enabling researchers to stay ahead of viral mutations and design vaccines that are resilient to genetic variability.(10,44) By addressing the limitations of current models and expanding their accessibility, AI can further enhance its role in combating emerging and re-emerging infectious diseases.(121)
Benefits and challenges of using AI in vaccine development
The integration of artificial intelligence (AI) into vaccine development has introduced groundbreaking advancements in efficiency, precision, and accessibility.(8) However, its implementation also brings significant challenges related to equity, bias, and ethical considerations. This section explores the major benefits and challenges associated with the use of AI in vaccine development.
Benefits
Reduction in Time and Cost of Research
One of the most significant advantages of AI in vaccine development is its ability to dramatically reduce the time and cost associated with research.(37,122) Traditional vaccine development pipelines often take years to complete due to labor-intensive processes such as antigen discovery, preclinical testing, and iterative optimization.(123,124) AI accelerates these processes by automating data analysis and modeling, enabling researchers to identify promising vaccine candidates in a fraction of the time.(123,125)
For example, during the COVID-19 pandemic, AI tools facilitated the rapid identification of the SARS-CoV-2 spike protein as a vaccine target and expedited the design of mRNA vaccines.(37) Pfizer-BioNTech and Moderna utilized AI-driven predictive models to optimize their vaccine candidates, achieving regulatory approval within one year—a timeline unprecedented in vaccine development history.(7,126) These advances not only save time but also reduce the financial burden of research, making vaccine development more feasible for a wider range of pathogens.
Increased Precision in Predicting Vaccine Candidates
AI excels at analyzing large and complex datasets, enabling greater precision in the identification of antigens, adjuvants, and other vaccine components.(122) Machine learning algorithms can process genomic, proteomic, and immunological data to identify epitopes that are highly likely to elicit immune responses.(69,127) This precision reduces the risk of failure during clinical trials, where many traditional vaccine candidates do not demonstrate sufficient efficacy.
For instance, tools like NetMHC and DeepVacPred have been used to predict MHC-binding epitopes with high accuracy, improving the selection of antigens for experimental validation.(128,129) Such capabilities minimize trial-and-error in the vaccine development process and contribute to higher success rates for candidate vaccines.
Potential for Personalizing Vaccines for Specific Regions or Populations
AI also holds great promise for the personalization of vaccines, allowing for tailored approaches to meet the needs of specific regions or populations.(37) By analyzing genomic and epidemiological data, AI models can predict how vaccine efficacy may vary across populations with different genetic backgrounds, immune profiles, or exposure risks.(130)
For example, regional variations in MHC genotypes have been linked to differences in immune responses to certain vaccines.(131) AI can address these disparities by designing vaccines optimized for regional populations, ensuring broader and more equitable protection.(10) This capability is especially important in addressing diseases with high geographic variability, such as malaria and dengue fever, where pathogen strains and immune responses can differ significantly between regions.
Challenges
Unequal Access to Advanced Technologies
Despite its transformative potential, the adoption of AI in vaccine development is often hindered by unequal access to advanced technologies.(132) Many low- and middle-income countries (LMICs) lack the computational infrastructure, technical expertise, and financial resources required to implement AI-driven approaches.(133) This disparity perpetuates global inequities in vaccine development and access, as LMICs remain dependent on external entities for vaccine production and distribution.(134)
Efforts to democratize AI tools, such as open-access platforms and collaborative research initiatives, have shown promise in addressing these disparities.(135) However, substantial investments in infrastructure and capacity building are still required to ensure equitable access to AI-driven vaccine development.
Risks of Bias in AI Models
AI models are only as good as the data on which they are trained, and biases in these datasets can lead to suboptimal or even harmful outcomes.(136) For instance, if training datasets disproportionately represent pathogens from specific regions or populations, the resulting models may fail to predict relevant antigens or adjuvants for underrepresented groups.(137) This bias can exacerbate health inequities by producing vaccines that are less effective for certain populations.(138)
To mitigate these risks, it is essential to prioritize the inclusion of diverse and representative datasets in AI training processes. Additionally, continuous validation of AI models against real-world data is crucial to ensure their reliability and generalizability across different contexts.
Ethical Challenges in the Use of AI in Vaccine Development
The integration of artificial intelligence into vaccine development introduces a host of ethical challenges that must be addressed to ensure equitable and responsible implementation. One prominent issue is the potential for algorithmic bias, which can arise from the underrepresentation of certain populations in training datasets. For instance, genomic data used to train AI models often disproportionately represent individuals from high-income countries, leading to predictive outputs that may fail to account for the genetic diversity present in low- and middle-income populations.(136) This bias could result in vaccines that are less effective or even entirely ineffective for underrepresented groups, exacerbating global health inequities.
Data privacy and security are additional ethical dilemmas. The use of large-scale genomic datasets for training AI models raises concerns about the potential misuse of sensitive information. While such datasets are invaluable for identifying immunogenic targets and optimizing vaccine efficacy, their collection and storage must comply with stringent privacy regulations to prevent unauthorized access or exploitation.(11,139) A notable example of this challenge is the risk of genomic data being used for discriminatory practices, such as denying insurance coverage based on genetic predispositions identified through AI analysis.
Another critical ethical consideration is the issue of equitable access to AI-driven vaccine technologies. The computational infrastructure and technical expertise required to implement AI in vaccine development are often concentrated in resource-rich settings, leaving low- and middle-income countries dependent on external entities for technological solutions.(133) This imbalance raises questions about ownership of innovations and the fair distribution of vaccines derived from AI-driven approaches. For example, during the COVID-19 pandemic, the unequal access to vaccines developed using advanced technologies underscored the urgency of addressing these disparities through international cooperation and technology transfer.(134)
Furthermore, the rapid adoption of AI in vaccine research necessitates robust regulatory frameworks to oversee its ethical use. The lack of standardized guidelines for AI applications in vaccinology can lead to inconsistent practices, where ethical considerations may be overshadowed by the urgency to produce results. For instance, debates surrounding the prioritization of vaccine candidates during pandemics highlight the tension between accelerating innovation and ensuring thorough ethical review processes.(120)
Addressing these ethical challenges requires a multi-faceted approach that combines the development of diverse and representative datasets, stringent data governance policies, and equitable investment in AI infrastructure. By prioritizing these efforts, researchers and policymakers can ensure that the benefits of AI in vaccine development are realized without compromising ethical principles or exacerbating existing disparities.
Ethical and Privacy Concerns in Genomic Data Use
The use of genomic data in AI-driven vaccine development raises significant ethical and privacy concerns. AI models often rely on large-scale genomic datasets to identify antigens and predict immune responses. However, the collection, storage, and analysis of such data can compromise individual and community privacy, particularly in settings with inadequate data protection regulations.(11,139)
Moreover, the potential for misuse of genomic data, such as discriminatory practices or unauthorized surveillance, highlights the need for stringent ethical guidelines and governance frameworks.(140) Implementing robust data anonymization techniques, obtaining informed consent from data contributors, and adhering to international data protection standards are essential to address these concerns.(141)
AI’s ability to revolutionize vaccine development is undeniable, offering substantial benefits in efficiency, precision, and personalization.(142) However, addressing challenges related to equitable access, bias, and ethical considerations is essential to maximize its potential and ensure that its advantages are distributed fairly across global populations.(143)
Prominent case studies
Artificial intelligence (AI) has profoundly influenced the development of vaccines across diverse disease contexts. This section highlights three pivotal case studies: the development of mRNA vaccines for SARS-CoV-2, the application of AI in malaria vaccine research, and the improvement of influenza vaccines through predictive modeling. Each case demonstrates the transformative impact of AI on vaccine design, development speed, and efficacy.
Case 1: Development of mRNA Vaccines for SARS-CoV-2
Role of AI in Identifying the Spike Protein as a Target
The COVID-19 pandemic underscored the urgency of developing effective vaccines in record time.(144) Central to this achievement was the application of AI in the rapid analysis of the SARS-CoV-2 genome.(145) Shortly after the virus’s genome was sequenced in January 2020, AI-driven computational tools were employed to identify the spike (S) protein as a primary antigenic target.(146,147) This protein, which facilitates viral entry into host cells via the ACE2 receptor, emerged as an optimal candidate for vaccine development due to its surface exposure and immunogenic properties.(148)
AI algorithms analyzed structural and functional data from SARS-CoV-2 and other coronaviruses, enabling researchers to pinpoint conserved regions of the spike protein, such as the receptor-binding domain (RBD).(149) This identification process, which traditionally could take months, was completed in mere days, significantly expediting downstream vaccine development.(150)
Impact on the Speed of Vaccine Development
The rapid identification of the spike protein facilitated the design of mRNA vaccines by companies such as Pfizer-BioNTech and Moderna. AI played a crucial role in optimizing the genetic sequences for the mRNA, ensuring efficient translation into spike protein antigens once administered. As a result, clinical trials for these vaccines began within months, culminating in emergency use authorization by the end of 2020.
This unprecedented speed was made possible by AI’s ability to integrate genomic, proteomic, and immunological data, enabling real-time simulations of vaccine efficacy and safety.(147) The success of these mRNA vaccines has established a new benchmark for vaccine development timelines, demonstrating the critical role of AI in pandemic preparedness.
Case 2: Application of AI in Malaria Vaccine Development
Identification of Promising Antigens Using AI Models
Malaria remains a significant global health burden, particularly in low- and middle-income countries (LMICs).(151) The complexity of Plasmodium parasites, which undergo multiple life stages and exhibit significant genetic variability, has hindered vaccine development efforts.(152) However, AI has emerged as a powerful tool in addressing these challenges by enabling the systematic identification of antigenic targets.(153)
Machine learning algorithms have been employed to analyze genomic and proteomic data from Plasmodium species, identifying conserved antigens that are critical for parasite survival and replication.(154) For instance, AI models have successfully predicted epitopes in the circumsporozoite protein (CSP), a key target for malaria vaccines, such as RTS,S/AS01, the first malaria vaccine approved by the World Health Organization (WHO).(155,156,157)
By streamlining the antigen discovery process, AI has facilitated the development of next-generation malaria vaccines that target multiple life stages of the parasite.(44) These advancements hold promise for improving vaccine efficacy and reducing the global burden of malaria.
Case 3: Predicting Influenza Virus Variants and Enhancing Vaccine Efficacy
How AI Has Improved Seasonal Vaccine Effectiveness
Influenza poses a unique challenge for vaccine development due to its rapid antigenic drift and shift, which result in the frequent emergence of new viral strains.(111) The efficacy of seasonal influenza vaccines depends on accurately predicting which strains will circulate in the upcoming season.(158) Traditionally, this process has relied on epidemiological surveillance and expert consensus, but it is often hindered by uncertainties in strain selection.
AI has revolutionized this process by enabling more accurate predictions of influenza variants.(48) Predictive models, such as those implemented by Nextstrain, use machine learning algorithms to analyze genomic data from global influenza surveillance networks.(94) These models identify mutation hotspots in hemagglutinin (HA) and neuraminidase (NA) proteins, which are key targets for vaccines.(159)
Incorporating AI into the strain selection process has improved the match between vaccine formulations and circulating strains, leading to higher efficacy rates for seasonal influenza vaccines.(10,160) Moreover, AI has facilitated the development of universal influenza vaccines by identifying conserved epitopes across diverse strains.(161) These innovations have the potential to reduce the global health and economic impact of influenza outbreaks.(162)
AI’s application in these case studies illustrates its transformative potential in addressing complex challenges in vaccine development. From enabling rapid responses to emerging pathogens like SARS-CoV-2, to tackling persistent diseases such as malaria and influenza, AI continues to reshape the landscape of vaccinology and public health.
Future directions in the use of AI in vaccinology
The future of vaccine development lies in leveraging artificial intelligence (AI) to overcome long-standing challenges, such as the design of universal vaccines, the personalization of vaccination strategies, and the proactive prevention of pandemics. These advancements have the potential to reshape global public health by addressing current limitations in vaccine efficacy, adaptability, and accessibility.
Development of Universal Vaccines
One of the most ambitious goals in vaccinology is the creation of universal vaccines that offer long-lasting protection across multiple strains or even entire pathogen families.(163) AI plays a critical role in this endeavor by identifying conserved antigens—regions of a pathogen’s genome or proteome that remain stable across different strains.(49) Machine learning algorithms analyze large-scale genomic data to pinpoint these conserved regions, which are less likely to mutate and thus provide a reliable basis for vaccine targets.(69,164)
For example, in influenza research, AI has been used to identify conserved epitopes in hemagglutinin (HA) and neuraminidase (NA) proteins, key targets for universal flu vaccines.(165) Similarly, AI is aiding the development of vaccines targeting conserved regions of HIV’s envelope protein, which could neutralize diverse viral strains.(112) These approaches hold the promise of reducing the need for frequent updates to vaccine formulations, thereby improving global vaccination efforts.
AI in Genetic Therapies and Personalized Vaccination
The integration of AI into genetic and personalized medicine is opening new frontiers in vaccination strategies. By analyzing individual genomic, proteomic, and immunological data, AI can predict how a specific person or population will respond to a given vaccine.(164) This capability is particularly valuable for diseases with highly variable immune responses, such as malaria or tuberculosis.(166)
AI-driven predictive models can optimize vaccine formulations and dosing regimens for individual patients, enhancing vaccine efficacy and reducing adverse reactions.(122) For example, AI tools are already being developed to predict variations in major histocompatibility complex (MHC) genotypes, which influence immune response diversity across populations.(167) These innovations pave the way for personalized vaccines tailored to individual immune profiles.
Role in Preparing for Future Pandemics
AI is also poised to play a pivotal role in global pandemic preparedness. Integrating AI into epidemiological surveillance systems can enable real-time monitoring of emerging pathogens and early detection of outbreaks.(168) Machine learning algorithms analyze patterns in genomic, clinical, and environmental data to identify potential zoonotic spillovers or hotspots of disease transmission.(169)
Additionally, AI can streamline the development of vaccines during pandemics by rapidly identifying antigenic targets and modeling immune responses.(37) These capabilities, demonstrated during the COVID-19 pandemic, will be instrumental in future public health crises, allowing for faster and more coordinated global responses.
CONCLUSIONS
Artificial intelligence (AI) has emerged as a transformative force in vaccine development, revolutionizing processes that were traditionally time-consuming and resource-intensive. By enhancing the precision and efficiency of vaccine research, AI has enabled significant advancements in addressing the challenges posed by emerging and re-emerging diseases. AI’s capacity to analyze vast datasets with unparalleled speed and accuracy has fundamentally transformed the field of vaccinology. By automating processes such as antigen identification, adjuvant optimization, and immune response simulation, AI has dramatically reduced the time and cost required for vaccine development.
During the COVID-19 pandemic, AI played a crucial role in the rapid identification of the SARS-CoV-2 spike protein as a vaccine target, facilitating the development of highly effective mRNA vaccines in under a year. Similarly, AI has shown its potential in addressing persistent challenges, such as identifying antigens for complex pathogens like Plasmodium in malaria or predicting the evolution of highly mutable viruses like influenza. Beyond speeding up vaccine development, AI has also enhanced the precision of these efforts, as demonstrated by its role in designing vaccines tailored to specific populations or predicting conserved antigens for universal vaccines. These advancements highlight AI’s importance in creating robust and adaptable solutions to pressing global health challenges.
The integration of AI into vaccine development has significant practical implications for improving global health equity. By reducing the cost and time required for vaccine production, AI can enable faster responses to disease outbreaks, particularly in resource-limited settings. AI’s ability to predict and simulate immune responses also allows for the development of vaccines tailored to the needs of specific populations, addressing disparities in vaccine efficacy due to genetic, geographic, or socioeconomic factors. However, realizing these benefits requires addressing barriers to equitable access. Many low- and middle-income countries (LMICs) lack the infrastructure and expertise necessary to implement AI-driven approaches. Investments in training, technology transfer, and open-access platforms are essential to ensure that the benefits of AI in vaccine development are distributed fairly across all regions. Initiatives fostering international collaboration and knowledge-sharing can further promote equity in health outcomes.
Looking ahead, AI’s potential in vaccinology will continue to expand, but its impact will depend on addressing several priorities. Enhancing the quality and representation of genomic and epidemiological data is critical, as AI models are only as effective as the datasets on which they are trained. Comprehensive and diverse datasets are essential to reduce biases and improve the generalizability of AI-driven predictions. Simultaneously, ethical and regulatory frameworks must be strengthened to address concerns surrounding data privacy and algorithmic bias. Developing robust governance practices for genomic data collection, storage, and use will be key to maintaining public trust and ensuring ethical applications.
Furthermore, investments in global infrastructure are necessary to build computational capacity and technical expertise in LMICs, enabling more equitable access to AI-driven vaccine development tools. Public-private partnerships and international collaborations will be instrumental in achieving this goal. AI’s demonstrated value during the COVID-19 pandemic underscores its critical role in pandemic preparedness. Integrating AI into global surveillance systems and proactively developing vaccines for high-risk pathogens will enable more efficient and coordinated responses to future public health crises.
By addressing these challenges and opportunities, AI can continue to revolutionize vaccine development. Its applications in accelerating research, improving precision, and enhancing accessibility underline its role as a cornerstone of modern vaccinology. With strategic investments and a commitment to equity, AI has the potential to enable a future where vaccines are developed more efficiently, are effective across diverse populations, and are accessible to all. This paradigm shift represents not only a technological advancement but also a profound opportunity to reshape global health for the better.
BIBLIOGRAPHIC REFERENCES
1. Chernak ED, Influenza A, Coronavirus N. Evolving and emerging threats. Public Health Emergencies: Case Studies, Competencies, and Essential Services of Public Health. 2021;226.
2. Morens DM, Fauci AS. Emerging pandemic diseases: how we got to COVID-19. Cell. 2020;182(5):1077–92.
3. Ghattas M, Dwivedi G, Lavertu M, Alameh MG. Vaccine technologies and platforms for infectious diseases: Current progress, challenges, and opportunities. Vaccines (Basel). 2021;9(12):1490.
4. Gebre MS, Brito LA, Tostanoski LH, Edwards DK, Carfi A, Barouch DH. Novel approaches for vaccine development. Cell. 2021;184(6):1589–603.
5. Gupta R, Srivastava D, Sahu M, Tiwari S, Ambasta RK, Kumar P. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers. 2021;25:1315–60.
6. Katwaroo AR, Adesh VS, Lowtan A, Umakanthan S. The diagnostic, therapeutic, and ethical impact of artificial intelligence in modern medicine. Postgrad Med J. 2024;100(1183):289–96.
7. Farahani AF, Kasraei N. Evaluating the Impact of Artificial Intelligence on Vaccine Development: Lessons Learned from the COVID-19 Pandemic. medRxiv. 2024;2010–24.
8. Kannan S, Subbaram K, Faiyazuddin M. Artificial intelligence in vaccine development: Significance and challenges ahead. In: A Handbook of Artificial Intelligence in Drug Delivery. Elsevier; 2023. p. 467–86.
9. Thalange A V, Patil AR, Athavale VA. A Review of Artificial Intelligence and Machine Learning for Vaccine Research. In: The International Conference on Recent Innovations in Computing. Springer; 2023. p. 85–101.
10. Olawade DB, Teke J, Fapohunda O, Weerasinghe K, Usman SO, Ige AO, et al. Leveraging artificial intelligence in vaccine development: A narrative review. J Microbiol Methods. 2024;106998.
11. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44–56.
12. Lawrence P, Heung M, Nave J, Henkel C, Escudero-Pérez B. The natural virome and pandemic potential: Disease X. Curr Opin Virol. 2023;63:101377.
13. Paul J. Introduction to Infectious Diseases. In: Disease Causing Microbes. Springer; 2024. p. 1–63.
14. Sánchez CA, Venkatachalam‐Vaz J, Drake JM. Spillover of zoonotic pathogens: A review of reviews. Zoonoses Public Health. 2021;68(6):563–77.
15. Rodriguez-Morales AJ, Paniz-Mondolfi AE, Faccini-Martínez ÁA, Henao-Martínez AF, Ruiz-Saenz J, Martinez-Gutierrez M, et al. The constant threat of zoonotic and vector-borne emerging tropical diseases: living on the edge. Vol. 2, Frontiers in tropical diseases. Frontiers Media SA; 2021. p. 676905.
16. Spencer JNH, Marasco D, Eichinger M. Planning for Emerging Infectious Disease Pandemics: Definitions, the role of planners, and learning from the avian influenza outbreak of 2004–2005. Journal of the American planning association. 2022;88(1):113–26.
17. Bhanye AS, Bhanye JI. Urban health in the 21st century: Exploring innovative measures to reduce the spread of communicable diseases. Developments in Environmental Science. 2024;15:503–27.
18. Wainaina M, Wasonga J, Cook EAJ. Epidemiology of human and animal leptospirosis in Kenya: A systematic review and meta-analysis of disease occurrence, serogroup diversity and risk factors. PLoS Negl Trop Dis. 2024;18(9):e0012527.
19. Kaslow RA, Bell DM. Epidemiology and Control: From Principles to Pandemics. In: Viral Infections of Humans: Epidemiology and Control. Springer; 2022. p. 1–80.
20. Matić Z, Šantak M. Current view on novel vaccine technologies to combat human infectious diseases. Appl Microbiol Biotechnol. 2022;106:25–56.
21. Meganck RM, Baric RS. Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nat Med. 2021;27(3):401–10.
22. Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. New England journal of medicine. 2020;382(21):1969–73.
23. Chowdhury K, Ahmad R, Sinha S, Dutta S, Haque M. Multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) among children: Where we stand now. Cureus. 2023;15(2).
24. Emane AKA, Guo X, Takiff HE, Liu S. Highly transmitted M. tuberculosis strains are more likely to evolve MDR/XDR and cause outbreaks, but what makes them highly transmitted? Tuberculosis. 2021;129:102092.
25. Sun-Waterhouse DX, Chen XY, Liu ZH, Waterhouse GI, Kang WY. Transformation from traditional medicine-food homology to modern food-medicine homology. Food & Medicine Homology. 2024;1(1):9420014.
26. Redford KH, Adams WM. Strange natures: Conservation in the era of synthetic biology. Yale University Press; 2021.
27. Khan M, Adil SF, Alkhathlan HZ, Tahir MN, Saif S, Khan M, et al. COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules. 2020;26(1):39.
28. Vecchio R, Gentile L, Tafuri S, Costantino C, Odone A. Exploring future perspectives and pipeline progression in vaccine research and development. Ann Ig. 2024;36(4):446–61.
29. Sharma O, Sultan AA, Ding H, Triggle CR. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front Immunol. 2020;11:585354.
30. Zhuang L, Ye Z, Li L, Yang L, Gong W. Next-generation TB vaccines: progress, challenges, and prospects. Vaccines (Basel). 2023;11(8):1304.
31. Patel MN, Patel AJ, Nandpal MN, Raval MA, Patel RJ, Patel AA, et al. Advancing against drug-resistant tuberculosis: an extensive review, novel strategies and patent landscape. Naunyn Schmiedebergs Arch Pharmacol. 2024;1–24.
32. Hosseinian SA, Hajati MH. A comprehensive review of the zoonotic potential of avian influenza viruses: a globally circulating threat to pandemic influenza in human. Journal of Zoonotic Diseases. 2024;
33. Gangopadhayya A, Bhukya PL. Factors Contributing to the Emergence of Viral Diseases. In: Emerging Human Viral Diseases, Volume I: Respiratory and Haemorrhagic Fever. Springer; 2023. p. 3–69.
34. Callaway E. Delta coronavirus variant: scientists brace for impact. Nature. 2021;595(7865):17–8.
35. Rubinstein M, Makhon A, Losev Y, Valenci GZ, Gatt YE, Margalit H, et al. Prolonged survival of a patient with active MDR-TB HIV co-morbidity: insights from a Mycobacterium tuberculosis strain with a unique genomic deletion. Front Med (Lausanne). 2023;10:1292665.
36. Abhinand CS, Prabhakaran AA, Krishnamurthy A, Raju R, Keshava Prasad TS, Nair AS, et al. SARS-CoV-2 variants infectivity prediction and therapeutic peptide design using computational approaches. J Biomol Struct Dyn. 2023;41(20):11166–77.
37. Ghosh A, Larrondo-Petrie MM, Pavlovic M. Revolutionizing vaccine development for COVID-19: a review of AI-based approaches. Information. 2023;14(12):665.
38. ElSherif M, Halperin SA. Benefits of combining Molecular Biology and Controlled Human Infection Model methodologies in advancing Vaccine Development. J Mol Biol. 2023;168322.
39. Hodgson J. The pandemic pipeline. Nat Biotechnol. 2020;38(5):523–32.
40. Ng’uni T, Chasara C, Ndhlovu ZM. Major scientific hurdles in HIV vaccine development: historical perspective and future directions. Front Immunol. 2020;11:590780.
41. Feldmann-Jensen S, DPPD MPH, O’Sullivan T. Informing Adaptation with Lessons Learned from Key 21st Century Infectious Disease Outbreaks. Current and Emerging Trends in the Management of International Disasters. 2024;
42. Ding C, Liu X, Yang S. The value of infectious disease modeling and trend assessment: a public health perspective. Expert Rev Anti Infect Ther. 2021;19(9):1135–45.
43. Sarley D, Hwang A, Hall BF, Ford A, Giersing B, Kaslow DC, et al. Accelerating access for all through research and innovation in immunization: Recommendations from Strategic Priority 7 of the Immunization Agenda 2030. Vaccine. 2022;
44. Aribi M. Development: From Traditional to Modern Approaches. New Topics in Vaccine Development. 2024;3.
45. Van Tilbeurgh M, Lemdani K, Beignon AS, Chapon C, Tchitchek N, Cheraitia L, et al. Predictive markers of immunogenicity and efficacy for human vaccines. Vaccines (Basel). 2021;9(6):579.
46. Zhang WY, Zheng XL, Coghi PS, Chen JH, Dong BJ, Fan XX. Revolutionizing adjuvant development: harnessing AI for next-generation cancer vaccines. Front Immunol. 2024;15:1438030.
47. Ananya, Panchariya DC, Karthic A, Singh SP, Mani A, Chawade A, et al. Vaccine design and development: Exploring the interface with computational biology and AI. Int Rev Immunol. 2024;1–20.
48. Zhao AP, Li S, Cao Z, Hu PJH, Wang J, Xiang Y, et al. AI for science: predicting infectious diseases. Journal of Safety Science and Resilience. 2024;
49. Vashisht V, Vashisht A, Mondal AK, Farmaha J, Alptekin A, Singh H, et al. Genomics for emerging pathogen identification and monitoring: Prospects and obstacles. BioMedInformatics. 2023;3(4):1145–77.
50. Farzan R. Artificial intelligence in Immuno-genetics. Bioinformation. 2024;20(1):29.
51. Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP. Introduction to machine learning, neural networks, and deep learning. Transl Vis Sci Technol. 2020;9(2):14.
52. De Groot AS, Moise L, Terry F, Gutierrez AH, Hindocha P, Richard G, et al. Better epitope discovery, precision immune engineering, and accelerated vaccine design using immunoinformatics tools. Front Immunol. 2020;11:442.
53. Parvizpour S, Pourseif MM, Razmara J, Rafi MA, Omidi Y. Epitope-based vaccine design: a comprehensive overview of bioinformatics approaches. Drug Discov Today. 2020;25(6):1034–42.
54. Khan MA, Amin A, Farid A, Ullah A, Waris A, Shinwari K, et al. Recent advances in genomics-based approaches for the development of intracellular bacterial pathogen vaccines. Pharmaceutics. 2022;15(1):152.
55. Bhattacharjee B, Bezbaruah R, Rynjah D, Newar A, Valu D, Ahmed N, et al. Proteogenomics and immunopeptidomics in the development of advanced vaccines. In: Advanced Vaccination Technologies for Infectious and Chronic Diseases. Elsevier; 2024. p. 455–75.
56. Angaitkar P, Janghel RR, Sahu TP. DL-TCNN: Deep Learning-based Temporal Convolutional Neural Network for prediction of conformational B-cell epitopes. 3 Biotech. 2023;13(9):297.
57. Dhanushkumar T, Santhosh ME, Selvam PK, Rambabu M, Dasegowda KR, Vasudevan K, et al. Advancements and hurdles in the development of a vaccine for triple-negative breast cancer: A comprehensive review of multi-omics and immunomics strategies. Life Sci. 2023;122360.
58. Liu Y, Ouyang X hui, Xiao ZX, Zhang L, Cao Y. A review on the methods of peptide-MHC binding prediction. Curr Bioinform. 2020;15(8):878–88.
59. Zhang Y, Mastouri M, Zhang Y. Accelerating drug discovery, development, and clinical trials by artificial intelligence. Med. 2024;
60. Bukhari SNH, Jain A, Haq E, Mehbodniya A, Webber J. Machine learning techniques for the prediction of B-cell and T-cell epitopes as potential vaccine targets with a specific focus on SARS-CoV-2 pathogen: A review. Pathogens. 2022;11(2):146.
61. Premkumar L, Segovia-Chumbez B, Jadi R, Martinez DR, Raut R, Markmann AJ, et al. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5(48):eabc8413.
62. Kowalzik F, Schreiner D, Jensen C, Teschner D, Gehring S, Zepp F. mRNA-based vaccines. Vaccines (Basel). 2021;9(4):390.
63. Bharadwaj KK, Srivastava A, Panda MK, Singh YD, Maharana R, Mandal K, et al. Computational intelligence in vaccine design against COVID-19. Computational intelligence methods in COVID-19: surveillance, prevention, prediction and diagnosis. 2021;311–29.
64. Alafif T, Tehame AM, Bajaba S, Barnawi A, Zia S. Machine and deep learning towards COVID-19 diagnosis and treatment: survey, challenges, and future directions. Int J Environ Res Public Health. 2021;18(3):1117.
65. Bastola R, Noh G, Keum T, Bashyal S, Seo JE, Choi J, et al. Vaccine adjuvants: smart components to boost the immune system. Arch Pharm Res. 2017;40:1238–48.
66. Zhao T, Cai Y, Jiang Y, He X, Wei Y, Yu Y, et al. Vaccine adjuvants: mechanisms and platforms. Signal Transduct Target Ther. 2023;8(1):283.
67. Elhassan Taha MM, Abdelwahab SI, Moni SS, Farasani A, Aljahdali IA, Oraibi B, et al. Nano-enhanced immunity: A bibliometric analysis of nanoparticles in vaccine adjuvant research. Hum Vaccin Immunother. 2024;20(1):2427464.
68. Kardani K, Bolhassani A, Namvar A. An overview of in silico vaccine design against different pathogens and cancer. Expert Rev Vaccines. 2020;19(8):699–726.
69. Bravi B. Development and use of machine learning algorithms in vaccine target selection. NPJ Vaccines. 2024;9(1):15.
70. Chowell D, Yoo SK, Valero C, Pastore A, Krishna C, Lee M, et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat Biotechnol. 2022;40(4):499–506.
71. Moni SS, Abdelwahab SI, Jabeen A, Elmobark ME, Aqaili D, Gohal G, et al. Advancements in vaccine adjuvants: The journey from alum to nano formulations. Vaccines (Basel). 2023;11(11):1704.
72. Pulendran B, S. Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–75.
73. Xu J, Liu W, Fan F, Zhang B, Sun C, Hu Y. Advances in nano-immunotherapy for hematological malignancies. Exp Hematol Oncol. 2024;13(1):57.
74. Pasupuleti D, Bagwe P, Ferguson A, Uddin MN, D’Souza MJ, Zughaier SM. Evaluating Nanoparticulate Vaccine Formulations for Effective Antigen Presentation and T-Cell Proliferation Using an In Vitro Overlay Assay. Vaccines (Basel). 2024;12(9):1049.
75. Russo G, Reche P, Pennisi M, Pappalardo F. The combination of artificial intelligence and systems biology for intelligent vaccine design. Expert Opin Drug Discov. 2020;15(11):1267–81.
76. Shin B, An G, Cockrell RC. Examining B-cell dynamics and responsiveness in different inflammatory milieus using an agent-based model. PLoS Comput Biol. 2024;20(1):e1011776.
77. Jamali Y. Modeling the Immune System Through Agent-based Modeling: A Mini-review. Immunoregulation. 2024;6(1):3–12.
78. Handel A, La Gruta NL, Thomas PG. Simulation modelling for immunologists. Nat Rev Immunol. 2020;20(3):186–95.
79. Hayawi K, Shahriar S, Alashwal H, Serhani MA. Generative AI and Large Language Models: A New Frontier in Reverse Vaccinology. Inform Med Unlocked. 2024;101533.
80. Chavda VP, Ghali ENHK, Balar PC, Chauhan SC, Tiwari N, Shukla S, et al. Protein subunit vaccines: Promising frontiers against COVID-19. Journal of Controlled Release. 2024;366:761–82.
81. Bali A, Bali N. Role of artificial intelligence in fast-track drug discovery and vaccine development for COVID-19. In: Novel AI and Data Science Advancements for Sustainability in the Era of COVID-19. Elsevier; 2022. p. 201–29.
82. Gao W, Liu J, Shtylla B, Venkatakrishnan K, Yin D, Shah M, et al. Realizing the promise of project optimus: challenges and emerging opportunities for dose optimization in oncology drug development. CPT Pharmacometrics Syst Pharmacol. 2024;13(5):691–709.
83. Poweleit EA, Vinks AA, Mizuno T. Artificial intelligence and machine learning approaches to facilitate therapeutic drug management and model-informed precision dosing. Ther Drug Monit. 2023;45(2):143–50.
84. Liu M, Li Q, Lin J, Lin Y, Hoffman E. Innovative trial designs and analyses for vaccine clinical development. Contemp Clin Trials. 2021;100:106225.
85. Chen X, He R, Chen X, Jiang L, Wang F. Optimizing dose-schedule regimens with bayesian adaptive designs: opportunities and challenges. Front Pharmacol. 2023;14:1261312.
86. Guarra F, Colombo G. Computational Methods in Immunology and Vaccinology: Design and Development of Antibodies and Immunogens. J Chem Theory Comput. 2023;19(16):5315–33.
87. Ortiz JPH, Osorio JE. One Health and Engineering: using engineering to further pave the roadmap towards global health security, pandemic preparedness, and personalized medicine. DYNA: revista de la Facultad de Minas Universidad Nacional de Colombia Sede Medellín. 2023;90(230):22–8.
88. Danchin A. Artificial intelligence‐based prediction of pathogen emergence and evolution in the world of synthetic biology. Microb Biotechnol. 2024;17(10):e70014.
89. Domingo E, García-Crespo C, Lobo-Vega R, Perales C. Mutation rates, mutation frequencies, and proofreading-repair activities in RNA virus genetics. Viruses. 2021;13(9):1882.
90. Kwok AJ, Mentzer A, Knight JC. Host genetics and infectious disease: new tools, insights and translational opportunities. Nat Rev Genet. 2021;22(3):137–53.
91. Al-Amran FG, Hezam AM, Rawaf S, Yousif MG. Genomic Analysis and Artificial Intelligence: Predicting Viral Mutations and Future Pandemics. arXiv preprint arXiv:230915936. 2023;
92. Nawaz MS, Fournier-Viger P, Shojaee A, Fujita H. Using artificial intelligence techniques for COVID-19 genome analysis. Applied Intelligence. 2021;51:3086–103.
93. Mohanty E, Mohanty A. Role of artificial intelligence in peptide vaccine design against RNA viruses. Inform Med Unlocked. 2021;26:100768.
94. Bernasconi A. The opportunity of data-driven services for viral genomic surveillance. In: 2023 IEEE International Conference on Service-Oriented System Engineering (SOSE). IEEE; 2023. p. 172–81.
95. Wei CJ, Crank MC, Shiver J, Graham BS, Mascola JR, Nabel GJ. Next-generation influenza vaccines: opportunities and challenges. Nat Rev Drug Discov. 2020;19(4):239–52.
96. Dănăilă VR, Avram S, Buiu C. The applications of machine learning in HIV neutralizing antibodies research—A systematic review. Artif Intell Med. 2022;134:102429.
97. Hederman AP, Ackerman ME. Leveraging deep learning to improve vaccine design. Trends Immunol. 2023;44(5):333–44.
98. Irvine EB, Reddy ST. Advancing antibody engineering through synthetic evolution and machine learning. The Journal of Immunology. 2024;212(2):235–43.
99. Elste J, Saini A, Mejia-Alvarez R, Mejía A, Millán-Pacheco C, Swanson-Mungerson M, et al. Significance of Artificial Intelligence in the Study of Virus–Host Cell Interactions. Biomolecules. 2024;14(8):911.
100. Ghosh A, Pavlovic M, Larrondo-Petrie MM. Rational Vaccine Design for SARS-CoV-2 Virus—A Systematic Review. 2023;
101. Taft JM, Weber CR, Gao B, Ehling RA, Han J, Frei L, et al. Deep mutational learning predicts ACE2 binding and antibody escape to combinatorial mutations in the SARS-CoV-2 receptor-binding domain. Cell. 2022;185(21):4008–22.
102. Scarpa F, Casu M. Genomics and Bioinformatics in One Health: Transdisciplinary Approaches for Health Promotion and Disease Prevention. Int J Environ Res Public Health. 2024;21(10):1337.
103. Ling-Hu T, Rios-Guzman E, Lorenzo-Redondo R, Ozer EA, Hultquist JF. Challenges and opportunities for global genomic surveillance strategies in the COVID-19 era. Viruses. 2022;14(11):2532.
104. Padhi A, Agarwal A, Saxena SK, Katoch CDS. Transforming clinical virology with AI, machine learning and deep learning: a comprehensive review and outlook. Virusdisease. 2023;34(3):345–55.
105. Bedi R, Bayless NL, Glanville J. Challenges and progress in designing broad-spectrum vaccines against rapidly mutating viruses. Annu Rev Biomed Data Sci. 2023;6(1):419–41.
106. Aminu RF, Emurotu MO, Mofolorunsho KC. Applications of Molecular Phylogeny in Disease Diagnosis. Int J Res Virol. 2024;1(1):1–8.
107. Ochsenreiter RW. Algorithmic Approaches on Structural RNA Evolution in Viruses. 2023;
108. Kushwaha SK, Kesarwani V, Choudhury S, Gandhi S, Sharma S. SARS-CoV-2 transcriptome analysis and molecular cataloguing of immunodominant epitopes for multi-epitope based vaccine design. Genomics. 2020;112(6):5044–54.
109. Rastogi A, Gautam S, Kumar M. Bioinformatic elucidation of conserved epitopes to design a potential vaccine candidate against existing and emerging SARS-CoV-2 variants of concern. Heliyon. 2024;10(15).
110. Giurgea LT, Morens DM, Taubenberger JK, Memoli MJ. Influenza neuraminidase: a neglected protein and its potential for a better influenza vaccine. Vaccines (Basel). 2020;8(3):409.
111. Biswas A, Chakrabarti AK, Dutta S. Current challenges: from the path of “original antigenic sin” towards the development of universal flu vaccines: flu vaccine efficacy encounters significant hurdles from pre-existing immunity of the host suggesting assessment of host immunity before vaccination. Int Rev Immunol. 2020;39(1):21–36.
112. Mahomed S. Broadly neutralizing antibodies for HIV prevention: a comprehensive review and future perspectives. Clin Microbiol Rev. 2024;e00152-22.
113. Brisse M, Vrba SM, Kirk N, Liang Y, Ly H. Emerging concepts and technologies in vaccine development. Front Immunol. 2020;11:583077.
114. Prabhod KJ. The Role of Machine Learning in Genomic Medicine: Advancements in Disease Prediction and Treatment. Journal of Deep Learning in Genomic Data Analysis. 2022;2(1):1–52.
115. Montesinos-López OA, Montesinos-López A, Pérez-Rodríguez P, Barrón-López JA, Martini JWR, Fajardo-Flores SB, et al. A review of deep learning applications for genomic selection. BMC Genomics. 2021;22:1–23.
116. Loewa A, Feng JJ, Hedtrich S. Human disease models in drug development. Nature reviews bioengineering. 2023;1(8):545–59.
117. Baquero F, Martinez JL, F. Lanza V, Rodríguez-Beltrán J, Galán JC, San Millán A, et al. Evolutionary pathways and trajectories in antibiotic resistance. Clin Microbiol Rev. 2021;34(4):e00050-19.
118. Williams D, Hornung H, Nadimpalli A, Peery A. Deep learning and its application for healthcare delivery in low and middle income countries. Front Artif Intell. 2021;4:553987.
119. Naseem M, Akhund R, Arshad H, Ibrahim MT. Exploring the potential of artificial intelligence and machine learning to combat COVID-19 and existing opportunities for LMIC: a scoping review. J Prim Care Community Health. 2020;11:2150132720963634.
120. Ebulue CC, Ekkeh OV, Ebulue OR, Ekesiobi CS. Leveraging machine learning for vaccine distribution in resource-limited settings: A synthesis of approaches. International Medical Science Research Journal. 2024;4(5):544–57.
121. Srivastava V, Kumar R, Wani MY, Robinson K, Ahmad A. Role of artificial intelligence in early diagnosis and treatment of infectious diseases. Infect Dis. 2024;1–26.
122. Abbasi N, Nizamullah FNU, Zeb S. AI IN HEALTHCARE: USING CUTTING-EDGE TECHNOLOGIES TO REVOLUTIONIZE VACCINE DEVELOPMENT AND DISTRIBUTION. JURIHUM: Jurnal Inovasi dan Humaniora. 2023;1(1):17–29.
123. Prajapati RN, Bhushan B, Singh K, Chopra H, Kumar S, Agrawal M, et al. Recent Advances in Pharmaceutical Design: Unleashing the Potential of Novel Therapeutics. Curr Pharm Biotechnol. 2024;25(16):2060–77.
124. Khuat TT, Bassett R, Otte E, Grevis-James A, Gabrys B. Applications of machine learning in biopharmaceutical process development and manufacturing: Current trends, challenges, and opportunities. arXiv preprint arXiv:231009991. 2023;
125. Sharma A, Virmani T, Pathak V, Sharma A, Pathak K, Kumar G, et al. Artificial intelligence‐based data‐driven strategy to accelerate research, development, and clinical trials of COVID vaccine. Biomed Res Int. 2022;2022(1):7205241.
126. Rappuoli R, Alter G, Pulendran B. Transforming vaccinology. Cell. 2024;187(19):5171–94.
127. Pertseva M, Gao B, Neumeier D, Yermanos A, Reddy ST. Applications of machine and deep learning in adaptive immunity. Annu Rev Chem Biomol Eng. 2021;12(1):39–62.
128. Bansal H, Aggarwal N. 19 Artificial Techniques Intelligenceto Design Epitope-Mapped. Handbook of AI-Based Models in Healthcare and Medicine: Approaches, Theories, and Applications. 2024;378.
129. Bansal H, Aggarwal N. Artificial Intelligence Techniques to Design Epitope-Mapped Vaccines and Diagnostics for Emerging Pathogens. In: Handbook of AI-Based Models in Healthcare and Medicine. CRC Press; p. 378–96.
130. Malone B, Simovski B, Moliné C, Cheng J, Gheorghe M, Fontenelle H, et al. Artificial intelligence predicts the immunogenic landscape of SARS-CoV-2 leading to universal blueprints for vaccine designs. Sci Rep. 2020;10(1):22375.
131. Slieker RC, Warmerdam DO, Vermeer MH, van Doorn R, Heemskerk MHM, Scheeren FA. Reassessing human MHC-I genetic diversity in T cell studies. Sci Rep. 2024;14(1):7966.
132. Deng Z. Pandemic-Resilient Investment: Sustainable Knowledge Infrastructure for Medical AI. Journal of the Knowledge Economy. 2024;1–24.
133. Pillai AS. Artificial Intelligence in Healthcare Systems of Low-and Middle-Income Countries: Requirements, Gaps, Challenges, and Potential Strategies. International Journal of Applied Health Care Analytics. 2023;8(3):19–33.
134. Gleeson D, Townsend B, Tenni BF, Phillips T. Global inequities in access to COVID-19 health products and technologies: a political economy analysis. Health Place. 2023;83:103051.
135. Ritoré Á, Jiménez CM, González JL, Rejón-Parrilla JC, Hervás P, Toro E, et al. The role of Open Access Data in democratizing healthcare AI: A pathway to research enhancement, patient well-being and treatment equity in Andalusia, Spain. PLOS Digital Health. 2024;3(9):e0000599.
136. Norori N, Hu Q, Aellen FM, Faraci FD, Tzovara A. Addressing bias in big data and AI for health care: A call for open science. Patterns. 2021;2(10).
137. Schleiss MR, Crooks CM, Karthigeyan KP, Kruc RM, Otero CE, Wang HY, et al. Proceedings of the Conference “CMV Vaccine Development—How Close Are We?”(27–28 September 2023). MDPI; 2024.
138. Jean-Jacques M, Bauchner H. Vaccine distribution—equity left behind? JAMA. 2021;325(9):829–30.
139. Topol E. Deep medicine: how artificial intelligence can make healthcare human again. Hachette UK; 2019.
140. Balagurunathan Y, Sethuraman RR. An Analysis of Ethics-Based Foundation and Regulatory Issues for Genomic Data Privacy. Journal of The Institution of Engineers (India): Series B. 2024;1–11.
141. Adekugbe AP, Ibeh CV. Navigating ethical challenges in data management for US program development: best practices and recommendations. International Journal of Management & Entrepreneurship Research. 2024;6(4):1023–33.
142. Kaushik R, Kant R, Christodoulides M. Artificial intelligence in accelerating vaccine development-current and future perspectives. Frontiers in Bacteriology. 2023;2:1258159.
143. Oyeniran CO, Adewusi AO, Adeleke AG, Akwawa LA, Azubuko CF. Ethical AI: Addressing bias in machine learning models and software applications. Computer Science & IT Research Journal. 2022;3(3):115–26.
144. Agampodi S, Mogeni OD, Chandler R, Pansuriya M, Kim JH, Excler JL. Global pandemic preparedness: learning from the COVID-19 vaccine development and distribution. Expert Rev Vaccines. 2024;23(1):761–72.
145. Chen J, Li K, Zhang Z, Li K, Yu PS. A survey on applications of artificial intelligence in fighting against COVID-19. ACM Computing Surveys (CSUR). 2021;54(8):1–32.
146. Casalino L, Dommer AC, Gaieb Z, Barros EP, Sztain T, Ahn SH, et al. AI-driven multiscale simulations illuminate mechanisms of SARS-CoV-2 spike dynamics. Int J High Perform Comput Appl. 2021;35(5):432–51.
147. Arora G, Joshi J, Mandal RS, Shrivastava N, Virmani R, Sethi T. Artificial intelligence in surveillance, diagnosis, drug discovery and vaccine development against COVID-19. Pathogens. 2021;10(8):1048.
148. Nejat R, Torshizi MF, Najafi DJ. S protein, ACE2 and host cell proteases in SARS-CoV-2 cell entry and infectivity; is soluble ACE2 a two blade sword? A narrative review. Vaccines (Basel). 2023;11(2):204.
149. Sanyal D, Banerjee S, Bej A, Chowdhury VR, Uversky VN, Chowdhury S, et al. An integrated understanding of the evolutionary and structural features of the SARS-CoV-2 spike receptor binding domain (RBD). Int J Biol Macromol. 2022;217:492–505.
150. Gote V, Bolla PK, Kommineni N, Butreddy A, Nukala PK, Palakurthi SS, et al. A comprehensive review of mRNA vaccines. Int J Mol Sci. 2023;24(3):2700.
151. Macarayan E, Papanicolas I, Jha A. The quality of malaria care in 25 low-income and middle-income countries. BMJ Glob Health. 2020;5(2):e002023.
152. Rajneesh, Tiwari R, Singh VK, Kumar A, Gupta RP, Singh AK, et al. Advancements and challenges in developing malaria vaccines: Targeting multiple stages of the parasite life cycle. ACS Infect Dis. 2023;9(10):1795–814.
153. Wong F, de la Fuente-Nunez C, Collins JJ. Leveraging artificial intelligence in the fight against infectious diseases. Science (1979). 2023;381(6654):164–70.
154. Musundi SD, Gitaka J, Kanoi BN. Identification of conserved cross-species B-cell linear epitopes in human malaria: A subtractive proteomics and immuno-informatics approach targeting merozoite stage proteins. Front Immunol. 2024;15:1352618.
155. Taddese S. Status in Malaria Vaccine Development: Basic aspects of Vaccine, Mechanism of actions, Vaccine pipelines, Stage oriented immune response ‘Challenges and Opportunities.’ Microbial journal. 2023;3(1).
156. Malik S, Waheed Y. Recent advances on vaccines against malaria: A review. Asian Pac J Trop Med. 2024;17(4):143–59.
157. Duffy PE. Current approaches to malaria vaccines. Curr Opin Microbiol. 2022;70:102227.
158. Ali ST, Cowling BJ. Influenza virus: tracking, predicting, and forecasting. Annu Rev Public Health. 2021;42(1):43–57.
159. Luczo JM, Spackman E. Epitopes in the HA and NA of H5 and H7 avian influenza viruses that are important for antigenic drift. FEMS Microbiol Rev. 2024;48(3).
160. Russell CA, Fouchier RAM, Ghaswalla P, Park Y, Vicic N, Ananworanich J, et al. Seasonal influenza vaccine performance and the potential benefits of mRNA vaccines. Hum Vaccin Immunother. 2024;20(1):2336357.
161. Rcheulishvili N, Mao J, Papukashvili D, Liu C, Wang Z, Zhao J, et al. Designing multi-epitope mRNA construct as a universal influenza vaccine candidate for future epidemic/pandemic preparedness. Int J Biol Macromol. 2023;226:885–99.
162. Sparrow E, Wood JG, Chadwick C, Newall AT, Torvaldsen S, Moen A, et al. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine. 2021;39(3):512–20.
163. Montero DA, Vidal RM, Velasco J, Carreño LJ, Torres JP, Benachi O MA, et al. Two centuries of vaccination: historical and conceptual approach and future perspectives. Front Public Health. 2024;11:1326154.
164. Lu G, Shan S, Zainab B, Ayaz Z, He J, Xie Z, et al. Novel vaccine design based on genomics data analysis: A review. Scand J Immunol. 2021;93(3):e12986.
165. Creytens S, Pascha MN, Ballegeer M, Saelens X, de Haan CAM. Influenza neuraminidase characteristics and potential as a vaccine target. Front Immunol. 2021;12:786617.
166. Mandala WL, Harawa V, Dzinjalamala F, Tembo D. The role of different components of the immune system against Plasmodium falciparum malaria: Possible contribution towards malaria vaccine development. Mol Biochem Parasitol. 2021;246:111425.
167. Matsuzaka Y, Yashiro R. Understanding and Therapeutic Application of Immune Response in Major Histocompatibility Complex (MHC) Diversity Using Multimodal Artificial Intelligence. BioMedInformatics. 2024;4(3):1835–64.
168. Munagandla VB, Dandyala SSV, Vadde BC. AI-Powered Cloud-Based Epidemic Surveillance System: A Framework for Early Detection. Revista de Inteligencia Artificial en Medicina. 2024;15(1):673–90.
169. Bag AK, Sengupta D. Computational frameworks for zoonotic disease control in Society 5.0: opportunities, challenges and future research directions. AI Soc. 2024;1–30.
FINANCING
The authors thank the Research and Development Department (DIDE) of the Technical University of Ambato (UTA) for funding the publication of the following research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Luis Fabián Salazar-Garcés.
Data curation: Rita Elizabeth Velastegui-Hernández, Diana Catalina Velastegui-Hernandez, Fabricio Alejandro Vasquez de la Bandera, Verónica Gabriela Salinas-Velastegui, Estefania Araceli Reyes-Rosero, Andrea Carolina Cevallos-Teneda, Andrea Alexandra Tufiño-Aguilar, Luis Felipe Contreras-Vásquez, Gabriela Sandoval.
Research: Rita Elizabeth Velastegui-Hernández, Diana Catalina Velastegui-Hernandez, Fabricio Alejandro Vasquez de la Bandera, Verónica Gabriela Salinas-Velastegui, Estefania Araceli Reyes-Rosero, Andrea Carolina Cevallos-Teneda, Andrea Alexandra Tufiño-Aguilar, Luis Felipe Contreras-Vásquez, Gabriela Sandoval, Luis Fabián Salazar-Garcés.
Project management: Rita Elizabeth Velastegui-Hernandez, Verónica Gabriela Salinas-Velastegui, Luis Fabián Salazar-Garcés.
Supervision: Rita Elizabeth Velastegui-Hernandez, Luis Felipe Contreras-Vásquez, Luis Fabián Salazar-Garcés.
Validation: Rita Elizabeth Velastegui-Hernandez, Verónica Gabriela Salinas-Velastegui, Luis Felipe Contreras-Vásquez, Luis Fabián Salazar-Garcés.
Drafting - original draft: Rita Elizabeth Velastegui-Hernandez, Luis Felipe Contreras-Vásquez, Luis Fabián Salazar-Garcés.
Writing - proofreading and editing: Rita Elizabeth Velastegui-Hernández, Diana Catalina Velastegui-Hernandez, Fabricio Alejandro Vasquez de la Bandera, Verónica Gabriela Salinas-Velastegui, Estefania Araceli Reyes-Rosero, Andrea Carolina Cevallos-Teneda, Andrea Alexandra Tufiño-Aguilar, Luis Felipe Contreras-Vásquez, Gabriela Sandoval, Luis Fabián Salazar-Garcés.