ORIGINAL ARTICLE
First report of fatal renal coccidiosis in a Biguá (Phalacrocorax brasilianus) in coinfection with nematodes from Argentina
Primer reporte de Coccidiosis renal fatal en un Biguá (Phalacrocorax brasilianus) en coinfección con nematodes de Argentina
Sergio Ivan Garijo1 ![]() *, María Cecilia Netri1
*, María Cecilia Netri1 ![]() *, Javier Origlia1
*, Javier Origlia1 ![]() *, Nancy Arias1
*, Nancy Arias1 ![]() *, Hugo Lopez Faray1
*, Hugo Lopez Faray1 ![]() *, Norberto Fabian Lopez1
*, Norberto Fabian Lopez1
![]() *, Maria Florencia
Unzaga1
*, Maria Florencia
Unzaga1![]() *, Miguel Victor Piscopo1
*, Miguel Victor Piscopo1![]() *
*
1Universidad Nacional de La Plata, Facultad de Ciencias Veterinarias, Catedra de Patología de Aves y Pilíferos. Buenos Aires, Argentina.
Cite AS: Garijo SI, Netri MC, Origlia J, Arias N, Lopez Faray H, Lopez NF, Unzaga MF, Piscopo MV. Primer reporte de Coccidiosis renal fatal en un Biguá (Phalacrocorax brasilianus) en coinfección con nematodes de Argentina. Salud Cienc. Tecnol. 2022;2:181. https://doi.org/10.56294/saludcyt2022181
Recieved: 27-11-2022 Revised: 16-12-2022 Accepted: 28-12-2022 Published: 29-12-2022
Editor: Lic.
Mabel Cecilia Bonardi ![]()
* English version made by: Lic. Mabel Cecilia Bonardi *
RESUMEN
Un espécimen de biguá (Phalacrocorax brasilianus) estando bajo condiciones de manejo clínico, luego de ser rescatado en estado de debilidad, experimentó una marcada mejoría general para luego sufrir un cambio abrupto hacia una evolución desfavorable hasta desenlace fatal en el curso de pocas horas debido a una falla renal aguda. El análisis de resultados de los estudios clínicos y de los hallazgos histopatológicos post mortem demostraron la presencia de una gran carga de nematodes gastrointestinales y de severa afección renal por Eimeriorinos. Estos últimos resultaron ser los causales de un extensivo daño renal suficiente para causar la falla renal aguda y considerarse la causa primaria de muerte. A lo largo de este trabajo se obtuvo una visión general de la patogenia y manifestaciones clínicas de la coccidiosis renal aviar, escasamente documentadas anteriormente. Este hallazgo coccidial nunca antes reportado en esta especie implica una alta probabilidad de registro de una nueva especie de Eimeria.
Palabras clave: Phalacrocorax (Nannopterum) Brasilianus; Parásitos Aviares; Coccidiosis Renal; Nematodes.
ABSTRACT
After being rescued from a state of weakness, a biguá (Phalacrocorax brasilianus) specimen under clinical management conditions underwent a remarkable general improvement before abruptly deteriorating until dying within a few hours as a result of acute renal failure. Clinical analysis and post-mortem histopathological findings demonstrated the presence of a large burden of gastrointestinal nematodes and severe renal involvement by Eimeriorina representatives. These latter were found to be the cause of extensive kidney damage sufficient to cause acute renal failure and to be considered the primary cause of death. Throughout this work, we obtained an overview of the pathogenesis and clinical manifestations of avian renal coccidiosis, which had been poorly documented previously. This coccidial finding, never before reported in this species, implies a high probability of recording a new species of Eimeria.
Keywords: Phalacrocorax (Nannopterum) Brasilianus; Avian Parasites; Renal Coccidiosis; Nematodes.
INTRODUCTION
The Biguá or Neotropical Cormorant, Phalacrocorax brasilianus (Gmelin, 1789) (Nannopterum brasilianus, Kennedy-Spencer 2014) (Suliformes: Phalacrocoracidae) is a coastal bird widely distributed in the American continent, from the southern United States to Tierra del Fuego in Argentina.(1)
Their populations are considered residents, since they do not move long distances, and due to their basically piscivorous diet, thus, it is unusual to find them far from water bodies.(1,2)
This bird is frequently observed in rivers, lagoons, and wetlands as well as along the sea coast in Buenos Aires and in the rest of South America.(3,4)
However, little and isolated information has been published on the parasitosis of this species, and these scarce studies refer mainly to metazoan parasites.(5,6,7) As a result, little is known about the parasitic protozoan that affects these birds today.
Enteric coccidiosis, caused by Eimeria and Isospora, is among the most common protozoan affections of wild and domestic birds. However, the development of these coccidia in extraintestinal locations other than the reticuloendothelial system or leukocytes in circulating blood has only been documented with species of the genus Eimeria. These reports on extraintestinal locations in Eimeridae are mainly related to Anseriformes and other wild avian hosts in aquatic environments (8,9,10,11,12,13) with only a few exceptions.(14) Moreover, given that most reports of these parasites in wild birds are based on specimens found dead, details about the signology during the course of the clinical picture are even scarcer.
In the present work, we aimed to describe the clinical evolution and post-mortem findings in a wild biguá naturally infected by renal coccidiosis and nematodes.
MÉTODOS
A specimen of Phalacrocorax brasilianus (Kennedy-Spencer 2014) was found by rescuers in the middle of an urban area, in Lanús, Province of Buenos Aires (34°42′S, 58°24′W) at an approximate distance of 6 km from the nearest coastal area in October 2019. Because of his weakened condition, he was captured and transferred to the care centre by rescuers. The clinical examination revealed wet plumage, hypothermia, alertness but limited responsiveness, and mild dehydration at the time of arrival. Additionally, the bird was also found lying prostrate at the bottom of the transport basket. First, the individual was given Dexamethasone (3 mg/kg) and vitamin B complex, as well as oral rehydration with a rehydration solution made specifically for human consumption (Gatorade). After two hours of treatment, he became stable, regaining his posture and attitude. He then started receiving recovery formula paste for dogs (Royal Canin Recovery) via esophageal gavage. Four hours later, he started eating on his own small fishes (Odontesthes sp.) for human consumption acquired locally. In addition, he begins excreting a large amount of concentrated urates and a small amount of faeces, all of which are signs of positive evolution. The presence of Phthiraptera ectoparasites was observed, so Fipronil spray was applied. The general condition and appetite significantly improved over the next 24 hours. The frequency and volume of faeces also increased, and stool samples were collected for coproparasitology. The individual began to decline rapidly on the third day after regurgitating a pellet of anisakid nematodes compressed with scales and other fish remains. He had lost his appetite and gradually became lethargic since then, until he fell from the perch in a comatose state only 8 hours after evacuating that pellet. As a result, he was kept under an infrared lamp and placed in an intraosseous catheter. Peripheral blood sample was obtained for smears and packed cell volume (PCV) determination. Fluids were given for six hours, with brief improvements after administering 25 % Glucose boluses, but stabilisation or diuresis weren’t achieved until the final cardiorespiratory arrest. During the stabilization attempts, Lactated Ringer’s Solution was administered as a continuous intraosseous infusion, along with Furosemide 1 mg/kg, Metoclopramide 2mg/kg, Dexamethasone 3 mg/kg. Furthermore, crushed fish with electrolyte solution were administered via esophageal catheter on two occasions, (15,16,17) which were later regurgitated. Anuria, lethargy, prostration, temporary disconnection of the sensorium, and slow and shallow breathing were the dominant signs, all of which indicated renal failure and severe metabolic imbalance.
Following direct examination in wet mounting, the faecal samples were analised using flotation techniques in Zinc Sulphate (33,6 %) and Sheep Solutions, as well as sedimentation in soapy water at 1 % (Trematodes). The blood smears were analised with rapid hematological staining (Color Fast-Biopack) and Giemsa staining. After performing the necropsy, samples were taken from esophagus, proventriculus/ventriculus, intestines, liver, pancreas, heart, larynx, trachea, syrinx, bronchus, lungs, kidneys, cloaca, muscle and spleen, which were fixed in 10 % formol, and then each sample was stained with Haematoxylin and Eosin (H&E). Following the observation of renal alterations, additional sections of this organ were stained with Periodic Acid-Schiff (PAS). Finally, despite attempts to extract coccidial DNA from kidney tissue for molecular studies, these efforts failed due to the long permanence of these sections in formol.
RESULTADOS
The PCV was 3,7 %, and in the analysis of peripheral blood smears no hemoparasites were found. Particularly, smears obtained from the leucocyte layer of the haematocrit tube (Giemsa staining) were negative.
The faecal samples processed by the aforementioned techniques confirmed the presence of large amounts of Contracaecum sp. eggs, few capillariid eggs and an abundant amount of unsporulated coccidian oocysts. In the direct wet mounts (without concentration processing) we also observed, a large number of urinary casts with the presence of hematic content (Figure 1).
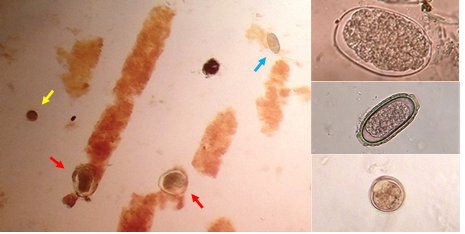
Figure 1. Photomicrographs of stool analysis. On the left image of direct examination in wet mount; an oocyst is observed (yellow arrow), two eggs of Contracaecum sp. (red arrows), a capillariid egg (blue arrow) and urinary casts. On the right enlarged images of the three findings present in the samples
DGross necropsy findings
The specimen did not show skin or muscle lesions. The dominant alterations at the opening of the coelomic cavity were the presence of a moderate amount of slightly cloudy serous pericardial fluid, hepatomegaly even more visible on the right lobe, a gallbladder with moderate repletion, and a prominent global gastric distention, since a characteristic of the Phalacrocoracidae is the absence of an externally visible division between the proventriculus and gizzard.(18)
After removing the heart and the digestive system, renal alterations and the presence of congestive areas in both lungs were observed. Kidneys were enlarged, had an irregular surface, slightly rounded lateral edges, and general discoloration with pale focal areas (Figure 2).
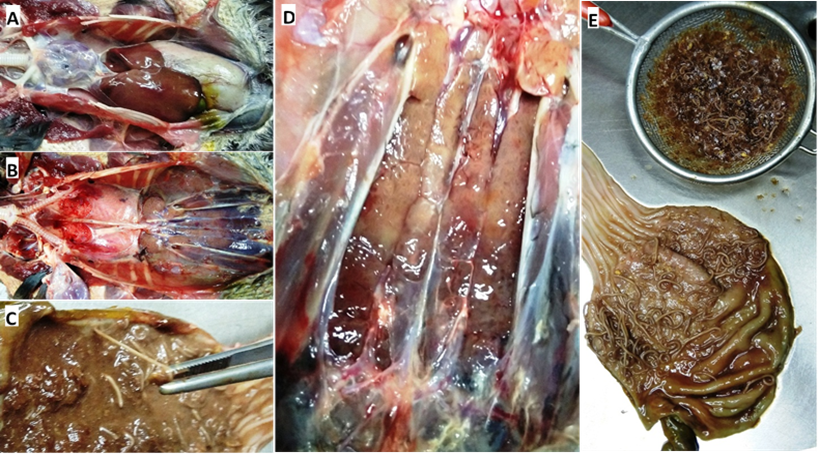
Figure 2. Most relevant macroscopic findings: A- General appearance upon opening of the coelomic cavity. B- View after removing the heart and the digestive tract, observe the coloration of the lungs and kidneys. C- Detail of the attachment of worms to the proventricular mucosa. D- Appearance of the kidneys in situ during necropsy. E- Aspect of the open stomach and esophagus showing the worms anchored to the mucosa after washing under the tap, as well as the contents recovered from its cavity
During the opening of the gastrointestinal tract, we found that the great distension in the proventriculus-gizzard was due to a large burden of Contracaecum sp. worms, filling almost the entire organ together with undigested fish remains. The vast majority of these worms were free in the organ’s lumen, and after washing under running water, it was clear that the worms attached to the wall were only present in the region of the proventricular glands. In the analysis of the contents of the gastrointestinal tract, no adult capillariids were recovered.
Histopathological findings
The most important tissue alterations were found in the kidney sections and showed several similarities with those described by Yabsley (19) and Abollo et al.(11) A severe and extensive nephrosis was observed, characterized by degeneration of the epithelium of the convoluted tubules, beginning with vacuolization in the basal region of the cells and displacement of the nuclei towards the apical border, initially reducing the lumen of the tubule, and ending with the detachment of the epithelial cells from their basement membrane, collapsing them towards the tubular lumen and causing their obliteration.
Distension of Bowman’s space was also observed, noticeable in certain sections of the slices, and blood extravasation around some intralobular vessels. Distension of collecting tubules, some of them showing total occlusion of the lumen with large accumulations of oocysts, as well as proteinaceous material and cellular debris. The presence of extremely distended tubules in the form of large masses, keeping the basement membrane intact; however, their markedly enlarged epithelial cells were filled with the multiplicative stages of Eimeria. Several tubules were observed with similar sizes but full of oocysts, while in others, different stages of development coexisted, including oocysts.
Scarce areas showed renal tubules without changes in their epithelial layers, and when they were present, they were found compressed by mechanical displacement due to the distension of those tubules altered around them. Despite all these changes in the normal architecture of the tissue, little or no inflammatory infiltration of the intertubular interstitium was observed, possibly because there was no disruption of the basement membranes of the tubules (Figure 3).
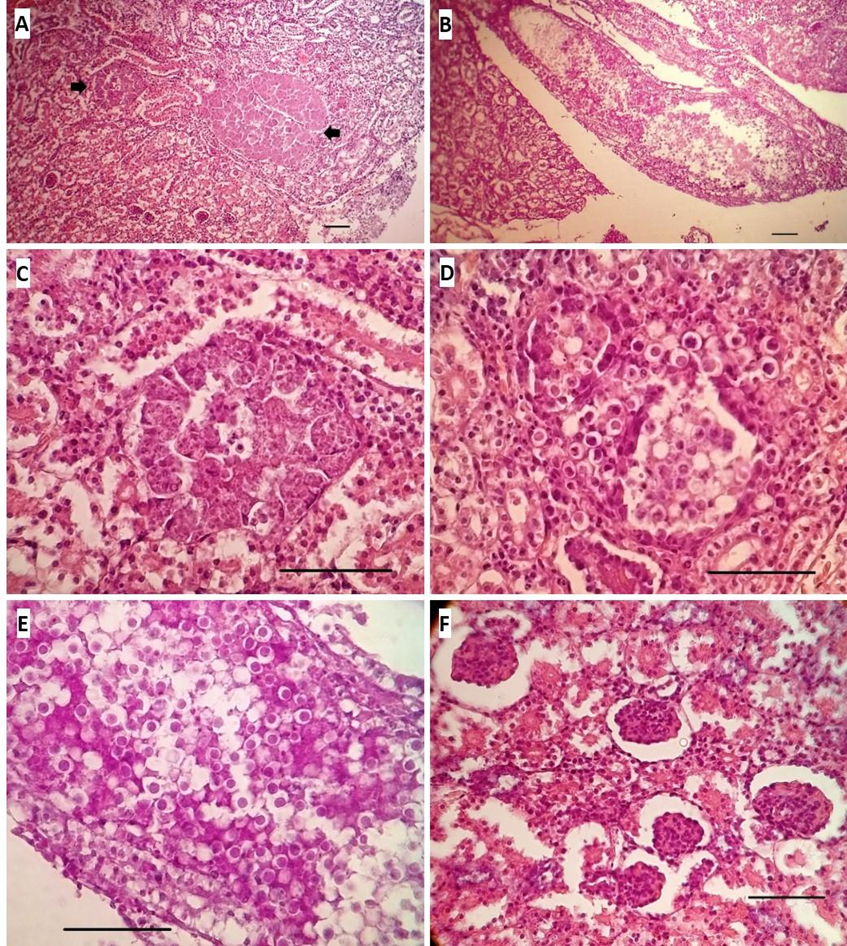
Figure 3. A- Generalized loss of normal renal architecture and collapse of tubular epithelia are observed; the arrows point to large masses of coccidial multiplicative stages (H&E 10x). B- Longitudinal section of a very distended main collecting duct due to the presence of a large number of oocysts, cellular debris and urates (PAS 10x). C- A sector of A showing a distended tubule with its epithelial cells housing multiple meronts and gamonts (H&E 40x). D- Transverse section of several contiguous tubules filled with oocysts occluding the lumen (H&E 40x). E- Image of the middle sector of the collecting tube shown in B with what could be considered an “embolus” of oocysts occluding the tubular lumen (PAS 40x). F- Detail of the distension in Bowman’s spaces; Note the expansion of some tubules and the resulting compression of other nearby ones (PAS 40x). Bar = 50 µ
The purpose of the present work is not the detailed description of the specific histopathological lesions of each apparatus or system, but the analysis of the alterations associated with renal coccidiosis and its clinical manifestations. However, it is opportune to briefly mention the alterations in other organs that could be relevant to establish the immunological health status in which the fatal clinical course took place.
In the lung tissue, we observed microthrombi in some blood vessels, perivascular edoema, and mild congestion in the capillary bed. When sections of the gastric apparatus were analysed, we observed distension of the lumen of multiple deep proventricular glands due to the presence of Contracaecum inside them, with haemorrhages and ulcerations on the luminal surface of the mucosa. In the small intestine, we found capillariids lodged in the thickness of the mucosa, some of them containing eggs, rupture and shortening and epithelial necrosis of the villi and necrosis in the epithelial lining of the crypts, and an accumulation of debris inside them; as well as a moderate inflammatory infiltrate in the adjacent connective tissue (Figure 4).
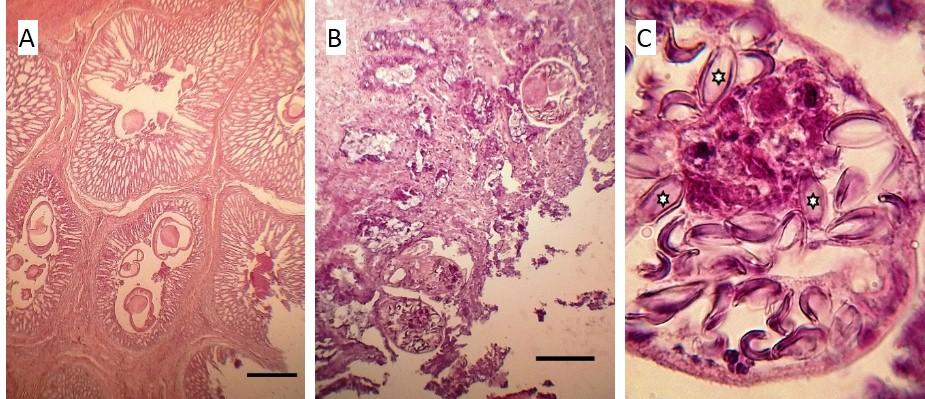
Figure 4. A- Full-thickness section of the proventricular mucosa, with the muscle layer in the upper left corner and the lumen of the organ in the lower right, highlighting the massive occupation of the proventricular glands with Concaracaecum worms (H&E 10x). B- Full-thickness section of the mucosa of the small intestine evidencing the disruption of the villi and the presence of capillarids at their base (H&E 10x). C- worms in the lower part of B, details of the content of double-walled elliptical eggs characteristic of Capillaria sp., three of which (stars) show their typical polar plugs (H&E Oil 100x). Bar = 100 µ
Finally, in liver slices, we observed mild Kupffer cell hyperplasia around some centrilobular venules, and the presence of isolated foci of acidophilic discoloration that showed loss of cytoplasmic and nuclear details of the hepatocyte sheets in the middle zone between portal areas and centrilobular veins.
The remaining organs sampled showed few or no significant changes for the case.
DISCUSSION
Although attempts to apply molecular techniques failed and it was not possible to sporulate oocysts in order to identify the genus or even species of the coccidia associated with this clinical case, based on the current status of knowledge about avian coccidiosis, we could infer that we are in the presence of an Eimeria sp. given the size and characteristics of the oocysts and that both the asexual and sexual multiplicative phases are completed in the same host. The only differential diagnosis that prevails as extraintestinal coccidiosis is the group of Isosporids formally called Atoxoplasma, however, their multiplicative stages outside the intestine only occur in phagocytic cells of the reticuloendothelial system and blood leukocytes,(20) which we did not observe in this case, in addition to the fact that they are only reported mainly in passerines. In light of the evidence previously discussed, the only possible genus would be Eimeria in this case.
In addition to the above, since the discovery of Eimeria truncata(21) —the first description of renal coccidiosis—previous reports on renal coccidiosis in cormorants indicate this affection was produced by Eimeria species.
One aspect of these parasites that has been extensively discussed is their true pathogenic potential, which is still debatable in wildlife because it is frequently an incidental finding in birds that have died from other causes or because it may not be possible to establish this coccidiosis as a primary cause of death. However, in this case, the acute clinical presentation and evolution associated with the histopathological finding demonstrate the pathogenic and lethal capacity of this coccidiosis. Besides confirming the presence of a high burden of nematodes, it is also clear that nematodes are a frequent finding in cormorants and other shorebirds that do not usually show any clinical signs. On the other hand, the same worms had probably been there for weeks or months, and yet the bird showed vigour, alertness and a voracious appetite 24 hours before the abrupt change in condition determined by acute renal failure. It is possible that for birds containing a certain number of renal meronts, with a certain rate of gametogony and rate of oocyst maturation, it may be physiologically tolerable below a certain threshold. If the threshold for oocyst maturation and oocyst shedding is exceeded due to changes in immune status, variability in the levels of impact on renal function and general homeostasis can be expected. Furthermore, with a significant amount of growing masses of meronts and gamonts scattered throughout the renal tissue compressing the surrounding parenchyma, it is expected that mechanical obliteration of the renal tubular system, as well as a large amount of cellular debris and proteinaceous material, will occur if these are “synchronised” due to some event of organic stress or significant changes in their environment causing massive maturation and discharge of oocysts. Consequently, there is an increase in intrtubular pressure proximal to these obstructions, as well as in the glomerular Bowman’s spaces, resulting in a dramatic reduction in normal renal filtration and resulting in loss of homeostasis. All of the above is evidenced throughout the findings presented in this report.
Regarding the Eimeriidae found in this work, due to the lack of reports of renal coccidiosis in South America, P. brasilianus is not a migratory species and only moves between coastal areas close to each other, and since renal coccidiosis has not been reported in this bird, there is a high probability of finding a new species of Eimeria.
Future research will be necessary to confirm or rule out the status of a new species of Eimeria, as well as to determine its distribution and its impact on populations of cormorants and perhaps other shorebirds.
REFERENCES
1. Frere E, Quintana F, Gandini P. Cormoranes de la costa patagónica: estado poblacional, ecología y conservación. El hornero 2005;20:35-52.
2. Conde-Tinco MA, Iannacone J. Bioecología del Phalacrocorax brasilianus (Gmelin, 1789)(Pelecaniformes: Phalacrocoracidae) en Sudamérica. The Biologist 2013;11:151-66. https://doi.org/10.24039/rtb2013111439.
3. Darrieu CA, Camperi AR, Piloni G, Bogado NR. Lista actualizada de las aves de la provincia de Buenos Aires. Buenos Aires: Fundación de Historia Natural «Félix de Azara»; 2013.
4. Iannacone J, Atasi M, Bocanegra T, Camacho M, Montes A, Santos S, et al. Diversidad de aves en el humedal Pantanos de Villa, Lima, Perú: periodo 2004-2007. Biota Neotrop 2010;10:295-304. https://doi.org/10.1590/S1676-06032010000200031.
5. Garbin L, Mattiucci S, Paoletti M, González-Acuña D, Nascetti G. Genetic and morphological evidences for the existence of a new species of Contracaecum (Nematoda: Anisakidae) parasite of Phalacrocorax brasilianus (Gmelin) from Chile and its genetic relationships with congeners from fish-eating birds. J Parasitol 2011;97:476-92. https://doi.org/10.1645/GE-2450.1.
6. Biolé FG, Guagliardo SE, Mancini MA, Tanzola RD, Salinas V, Morra G. Primer registro de Contracaecum australe (Nematoda: Anisakidae) en Phalacrocorax brasilianus (Aves: Phalacrocoracidae) de la región central de Argentina. BioScriba 2012;5:1-11.
7. González-Acuña D, Llanos-Soto S, Oyarzún-Ruiz P, Kinsella JM, Barrientos C, Thomas R, et al. Parasites of the Neotropic cormorant Nannopterum (Phalacrocorax) brasilianus (Aves, Phalacrocoracidae) in Chile. Rev Bras Parasitol Vet 2020;29:e003920. https://doi.org/10.1590/S1984-29612020049.
8. Wobeser G. Renal coccidiosis in mallard and pintail ducks. J Wildl Dis 1974;10:249-55. https://doi.org/10.7589/0090-3558-10.3.249.
9. Oksanen A. Mortality associated with renal coccidiosis in juvenile wild greylag geese (Anser anser anser). J Wildl Dis 1994;30:554-6. https://doi.org/10.7589/0090-3558-30.4.554.
10. Rossin A, Malizia AI. Relationship between helminth parasites and demographic attributes of a population of the subterranean rodent Ctenomys talarum (Rodentla: Octodontidae). J Parasitol 2002;88:1268-70. https://doi.org/10.1645/0022-3395(2002)088[1268:RBHPAD]2.0.CO;2.
11. Abollo E, Pascual S. Renal coccidiosis in the European cormorant Phalacrocorax aristotelis aristotelis from the Galician coast. Journal of the Marine Biological Association of the United Kingdom 2005;85:1017-9. https://doi.org/10.1017/S0025315405012075.
12. Atkinson CT, Thomas NJ, Hunter DB, editores. Parasitic Diseases of Wild Birds. Oxford, UK: Wiley-Blackwell; 2008. https://doi.org/10.1002/9780813804620.
13. Yabsley MJ, Gibbs SEJ. Description and phylogeny of a new species of Eimeria from double-crested cormorants (Phalacrocorax auritus) near Fort Gaines, Georgia. J Parasitol 2006;92:385-8. https://doi.org/10.1645/GE-592R.1.
14. Jankovsky JM, Brand M, Gerhold RW. Identification of a Novel Renal Coccidian (Apicomplexa: Eimeriidae) from the Great-Horned Owl ( Bubo virginianus ), USA. J Wildl Dis 2017;53:368-71. https://doi.org/10.7589/2016-06-132.
15. Beynon PH, Forbes NA, Harcourt-Brown NH. BSAVA manual of raptors, pigeons and waterfowl. Cheltenham, Gloucestershire: British Small Animal Veterinary Association; 1996.
16. Tully TN, Dorrestein GM, Jones AK. Handbook of avian medicine. 2nd ed. Edinburgh: Elsevier/Saunders; 2009.
17. Hawkins MG, Guzman DS-M, Beaufrère H, Lennox AM, Carpenter JW. Chapter 5 - Birds. En: Carpenter JW, Marion CJ, editores. Exotic Animal Formulary (Fifth Edition), W.B. Saunders; 2018, p. 167-375. https://doi.org/10.1016/B978-0-323-44450-7.00005-9.
18. Dziala-Szczepanczyk E, Wilczynska I. Morphology and morphometric analysis of selected elements of digestive tract in great cormorant Phalacrocorax carbo sinensis. Electronic Journal of Polish Agricultural Universities Series Biology 2010;13:25.
19. Yabsley MJ, Gottdenker NL, Fischer JR. Description of a new Eimeria sp. and associated lesions in the kidneys of double-crested cormorants (Phalocrocorax auritus). J Parasitol 2002;88:1230-3. https://doi.org/10.1645/0022-3395(2002)088[1230:DOANES]2.0.CO;2.
20. Barta JR, Schrenzel MD, Carreno R, Rideout BA. The Genus Atoxoplasma (Garnham 1950) as a Junior Objective Synonym of the Genus Isospora (Schneider 1881) Species Infecting Birds and Resurrection of Cystoisospora (Frenkel 1977) as the Correct Genus for Isospora Species Infecting Mammals. Journal of Parasitology 2005;91:726-7. https://doi.org/10.1645/GE-3341.1.
21. Railliet A, Lucet A. Une nouvelle maladie parasitaire de l’oie domestique, determinée par des coccidies. CR Soc Biol (Paris) 1890;42:293-4.
ACKNOWLEDGMENTS
Queremos agradecer a Erika Badura, de la Cátedra de Patología de Aves y Pilíferos, Facultad de Ciencias Veterinarias, Universidad Nacional de La Plata, por su entusiasta colaboración técnica en la elaboración de las preparaciones histológicas del presente trabajo.
FUNDING
Los autores no tienen financiamiento ni intereses económicos relevantes que declarar.
CONFLICTS OF INTEREST
Los autores no tienen conflictos de intereses para declarar que sean relevantes para el contenido de este artículo.
AUTHORSHIP CONTRIBUTION
Conceptualización: Sergio Ivan Garijo.
Redacción original y elaboración de imágenes: Sergio Ivan Garijo.
Revisión de redacción y edición: Sergio Ivan Garijo, Javier Origlia.
Técnicas moleculares: Javier Origlia.
Análisis de muestras histopatológicas: María Cecilia Netri, Nancy Arias, Hugo Lopez Faray, Norberto Fabian Lopez.
Supervisión: Norberto Fabian Lopez, Miguel Victor Piscopo.
Redacción – borrador original: Sergio Ivan Garijo.
Redacción – revisión y edición: Sergio Ivan Garijo, María Cecilia Netri, Javier Origlia, Nancy Arias, Hugo Lopez Faray, Norberto Fabian Lopez, Maria Florencia Unzaga, Miguel Victor Piscopo.